| Identification | More | [Name]
Iodotrimethylsilane | [CAS]
16029-98-4 | [Synonyms]
IODOTRIMETHYLSILANE
IODOTRIMETHYLSILYL IODIDE
TMIS
TRIMETHYLIODOSILANE
TRIMETHYLSILYL IODIDE
CT3610
iodotrimethyl-silan
Silane, iodotrimethyl-
trimethyliodosilane(tmis)
Iodotrmethylsilane
TMS iodide
Trimethyliodosilanestabilizedwithcoppergranules
IODOTRIMETHYLSILANE, STAB.
Iodotrimethylsilane, stabilised with copper, 95%
Iodotrimethylsilane, stabilized, 95-97%
Trimethylsilysiodide
IODOTRIMETHYLSILANE , STABILIZED WITH COPPER
IODO TRIMETHYL SILYL
trimethyiodosilane
Iodotrimethylsilane, 97%, stab. with copper | [EINECS(EC#)]
240-171-0 | [Molecular Formula]
C3H9ISi | [MDL Number]
MFCD00001028 | [Molecular Weight]
200.09 | [MOL File]
16029-98-4.mol |
| Chemical Properties | Back Directory | [Appearance]
Straw liquid | [Melting point ]
<0°C | [Boiling point ]
106 °C(lit.)
| [density ]
1.406 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.471(lit.)
| [Fp ]
−25 °F
| [storage temp. ]
−20°C
| [solubility ]
reacts | [form ]
Liquid | [color ]
Clear colorless to reddish | [Specific Gravity]
1.47 | [Water Solubility ]
reacts | [Hydrolytic Sensitivity]
8: reacts rapidly with moisture, water, protic solvents | [Sensitive ]
Moisture & Light Sensitive | [Detection Methods]
GC | [BRN ]
1731136 | [Stability:]
Moisture Sensitive (Reactive) | [InChIKey]
CSRZQMIRAZTJOY-UHFFFAOYSA-N | [CAS DataBase Reference]
16029-98-4(CAS DataBase Reference) | [NIST Chemistry Reference]
Iodotrimethylsilane(16029-98-4) | [Storage Precautions]
Store under inert gas;Light sensitive;Moisture sensitive;Heat sensitive | [EPA Substance Registry System]
16029-98-4(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
F,C | [Risk Statements ]
R11:Highly Flammable.
R14:Reacts violently with water.
R34:Causes burns. | [Safety Statements ]
S16:Keep away from sources of ignition-No smoking .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S43:In case of fire, use ... (indicate in the space the precise type of fire-fighting equipment. If water increases the risk add-Never use water) .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S25:Avoid contact with eyes . | [RIDADR ]
UN 2924 3/PG 2
| [WGK Germany ]
3
| [F ]
8-21 | [TSCA ]
Yes | [HazardClass ]
3 | [PackingGroup ]
II | [HS Code ]
29310095 |
| Hazard Information | Back Directory | [Chemical Properties]
Straw liquid | [Physical properties]
bp 106–109 °C; d 1.406 g cm?3; nD
20 1.4710;
fp ?31 °C. | [Uses]
Iodotrimethylsilane is used for the introduction of trimethylsilyl group in organic synthesis. It is also useful for gas chromatography analysis by converting alcohol into a silyl ether derivative, thereby making it more volatile than the original molecule. | [Application]
Iodotrimethylsilane can be used as a versatile reagent for the mild dealkylation of ethers, carboxylic esters, lactones, carbamates, acetals, phosphonate and phosphate esters; cleavage of epoxides, cyclopropyl ketones; conversion of vinyl phosphates to vinyl iodides; neutral nucleophilic reagent for halogen exchange reactions, carbonyl and conjugate addition reactions; use as a trimethylsilylating agent for formation of enol ethers, silyl imino esters, and N-silylenamines, alkyl, alkenyl and alkynyl silanes; Lewis acid catalyst for acetal formation, α- alkoxymethylation of ketones, for reactions of acetals with silyl enol ethers and allylsilanes; reducing agent for epoxides, enediones, α-ketols, sulfoxides, and sulfonyl halides; dehydrating agent for oximes. | [Preparation]
Iodotrimethylsilane is prepared by mixing equimolar amounts of chlorotrimethylsilane and sodium iodide in acetonitrile, reduces various benzylic alcohols to the corresponding phenylalkanes. Other have been reported for the preparation of Trimethylsilyl Iodide(TMS-I): chlorotrimethylsilane undergoes halogen exchange with either lithium iodide in CHCl3 or sodium iodide in MeCN, which allows in situ reagent formation. Alternatively, hexamethyldisilane reacts with iodine at 25–61°C to afford TMS-I with no byproducts.
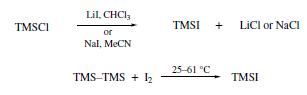 | [Reactions]
Trimethylsilyl iodide reacts under mild conditions in the absence of a catalyst with alkyl fluorides as well as with benzyl and tertiary alkyl chlorides and bromides to give good yields of alkyl iodides and the corresponding trimethylsilyl halides. | [General Description]
Iodotrimethylsilane is a multipurpose reagent used in various organic reactions. It is used for the dealkylation of few compounds like lactones, ethers, acetals, and carbamates and trimethylsilylating agent for the synthesis of silyl imino esters, alkyl and alkenyl silanes, etc. It also acts as a Lewis acid catalyst and as a reducing agent in many organic reactions. | [Synthesis]
A 250-ml., two-necked, round-bottomed flask equipped with a magnetic stirring bar, an addition funnel for solids, and a reflux condenser bearing a nitrogen inlet is charged with 5.6 g. (0.21 mole) of aluminum powder and 16.2 g. (0.100 mole) of hexamethyldisiloxane and purged with nitrogen. The mixture is stirred and heated with an oil bath at 60° as 50.8 g. (0.200 mole) of iodine is added slowly through the addition funnel over 55 minutes. The bath temperature is raised to ca. 140°, and the mixture is heated under reflux for 1.5 hours. The reflux condenser is removed, and the flask is equipped for distillation at atmospheric pressure. The bath temperature is gradually raised from 140° to 210°, and the clear, colorless distillate is collected, yielding 32.6–35.3 g. (82–88%) of iodotrimethylsilane, b.p. 106–109°. | [Purification Methods]
Add a little antimony powder and fractionate with this powder in the still. 20 1.470. Stabilise the distillate with 1% wt of Cu powder. [Eaborn J Chem Soc 3077 1950, Beilstein 4 IV 4009.] |
|
|