| Identification | Back Directory | [Name]
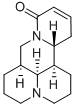
13,14-Didehydromatridin-15-one | [CAS]
145572-44-7 | [Synonyms]
SOPHOCARPINE
Matridine-13-ene-15-one
Sophocarpine Monohydrate
13,14-didehydromatridin-15-one
(6AS,11AR,11BR,11CS)-2,3,5,6,6A,7,11,11A,11B,11C-DECAHYDRO-1H,4H-3A,7A-DIAZA-BENZO[DE]ANTHRACEN-8-ONE
(41S,7aS,13aR,13bR)-2,3,41,6,7,7a,8,13,13a,13b-Decahydro-1H,5H,10H-dipyrido[2,1-f:3',2',1'-ij][1,6]naphthyridin-10-one hydrate | [Molecular Formula]
C15H22N2O | [MDL Number]
MFCD00274555 | [MOL File]
145572-44-7.mol | [Molecular Weight]
246.35 |
| Hazard Information | Back Directory | [Description]
A tetracyclic alkaloid present in Sophora species, this base crystallizes from H20
as the monohydrate, m.p. 54-5°C which becomes anhydrous in vacuo. It has
[α]18D - 29.44° (EtOH) and yields the following salts: hydrochloride, m.p. >
300°C; hydriodide, m.p. 277-9°C; aurichloride, m.p. l66-7°C; platinichloride,
m.p. 207-212°C (dec.); picrate, m.p. ISS-7°C and the methiodide, m.p. 200-2°C. Reduction of the base by electrolysis yields a crystalline, volatile base,
ClsH26N2, b.p. l53-4°C/5 mm; [α]D - 26.2° (EtOH) and giving a methiodide
with a melting point above 300°C. Attempted degradation by the Hofmann
process has proved unsuccessful. | [Uses]
Sophocarpine Monohydrate alleviates non-alcoholic steatohepatitis, which is a resultant effect of non-alcoholic fatty lifer disease. Admission of this compound results in a decrease in liver weight and serum lipids. Antiviral agent, Sophocarpine also displays a profound effect against coxsackievirus and therapeutical effects of viral myocarditis. | [References]
Orekhov, Proskurnina., Ber., 67,77 (1934)
Proskurnina, Kuzuvkov., Dokl. Akad. Nauk SSSR, 91, 1145 (1953)
Okuda et al., Chern. & Ind., 1326 (1962)
|
|
|