| Identification | More | [Name]
(ACETYLMETHYLENE)TRIPHENYLPHOSPHORANE | [CAS]
1439-36-7 | [Synonyms]
1-TRIPHENYLPHOSPHANYLIDENE-PROPAN-2-ONE
1-(TRIPHENYLPHOSPHORANYLIDENE)-2-PROPANONE
(ACETYLMETHYLENE)TRIPHENYLPHOSPHORANE
AURORA KA-1177
(METHYLCARBONYLMETHYLENE)TRIPHENYLPHOSPHORANE
triphenylphosphoranylidene-2-propanon
triphenylphosphoranylidene-2-propanone
1-(triphenylphosphoranylidene)acetone
2-Propanone, 1-(triphenylphosphoranylidene)-
2-Propanone, triphenylphosphoranylidene.
(Acetylylmethylene)triphenylphosphorane, 99 %
(2-Oxopropylidene)triphenylphosphorane
Triphenyl(2-oxopropylidene)phosphorane | [EINECS(EC#)]
215-878-2 | [Molecular Formula]
C21H19OP | [MDL Number]
MFCD00008774 | [Molecular Weight]
318.35 | [MOL File]
1439-36-7.mol |
| Chemical Properties | Back Directory | [Appearance]
white to light beige crystalline powder | [Melting point ]
203-205 °C (lit.) | [Boiling point ]
478.5±28.0 °C(Predicted) | [density ]
1.14 | [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
Soluble in chloroform. Slightly soluble in methanol. | [form ]
Powder | [color ]
White to off-white | [Stability:]
Stable. Combustible. Incompatible with strong oxidizing agents. | [Sensitive ]
Air Sensitive | [BRN ]
750077 | [CAS DataBase Reference]
1439-36-7(CAS DataBase Reference) |
| Safety Data | Back Directory | [Risk Statements ]
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes . | [WGK Germany ]
3
| [RTECS ]
UC3900000
| [HS Code ]
29319090 |
| Hazard Information | Back Directory | [Chemical Properties]
white to light beige crystalline powder | [Uses]
(Acetylmethylene)triphenylphosphorane is used as a Wittig reagent in synthetic chemistry, especially for the synthesis of functionalized pyrrolidines and cyclobutanones. It plays as a vital role in asymmetric allylboration for enantioselective synthesis of (+)-awajanomycin.It is also employed as a reactant in the preparation of 1,2-dioxanes with antitrypanosomal activity. Further, it is used in the preparation of amphibian pyrrolizidine alkaloids through allylic aminations and silicon-containing acyclic dienone musk odorants. In addition to this, it is involved in Domino Suzuki/Heck coupling reactions to prepare fluorenylidenes. | [Uses]
Wittig Reagent | [reaction suitability]
reaction type: C-C Bond Formation | [Synthesis]
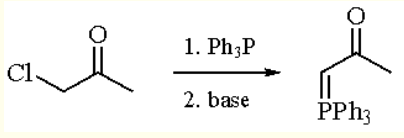
The preparative method of (Acetylmethylene)triphenylphosphorane: treatment of Triphenylphosphine with chloro- or Bromoacetone provides the phosphonium salt (eq 1). Deprotonation with a weak base such as bicarbonate in cold water provides the crude ylide which may by isolated by filtration.
| [storage]
Handling, Storage, and Precautions: may be handled in atmosphere for short periods; will decompose in water at pH > 9.
|
|
|