| Identification | More | [Name]
11a,16b,17,21-Tetrahydroxy-pregna-1,4-diene-3,20-dione | [CAS]
13951-70-7 | [Synonyms]
11a,16b,17,21-tetrahydroxy-pregna-1,4-diene-3,20-dione
11B,16A,17A,21-TETRAHYDROXYPREGNA-1,4-DIENE-3,20-DIONE
16A-HYDROXY-PREDNISOLONE
16-ALPHA-HYDROXY PREDNISOLONE
16α-hydroxy-prednisolone
11beta,16alpha,17,21-tetrahydroxypregna-1,4-diene-3,20-dione
16 Alpha Hydroxprednisolone
16A-HYDROXY-PREDNISONLONE
16α-HYDROXY-PREDNISONLONE
11,16a,17a,21-Tetrahydroxypregna-1,4-diene-3,20-dione
11β,16α,17,21-Tetrahydroxypregna-1,4-diene-3,20-dione
11β,16α,17α,21-Tetrahydroxypregna-1,4-diene-3,20-dione | [EINECS(EC#)]
237-731-1 | [Molecular Formula]
C21H28O6 | [MDL Number]
MFCD00872019 | [Molecular Weight]
376.44 | [MOL File]
13951-70-7.mol |
| Chemical Properties | Back Directory | [Appearance]
White Crystalline Solid | [Melting point ]
235-238°C | [Boiling point ]
591.5±50.0 °C(Predicted) | [density ]
1.38±0.1 g/cm3(Predicted) | [vapor pressure ]
0Pa at 25℃ | [storage temp. ]
Sealed in dry,2-8°C | [solubility ]
DMSO (Slightly), Methanol (Slightly, Heated) | [form ]
Solid | [pka]
11.93±0.70(Predicted) | [color ]
White to Light Yellow | [Water Solubility ]
815mg/L at 20℃ | [Usage]
A metabolite of Budesonide, an antiinflammatory agent | [LogP]
0.8 at 25℃ | [CAS DataBase Reference]
13951-70-7(CAS DataBase Reference) |
| Hazard Information | Back Directory | [Chemical Properties]
White Crystalline Solid | [Uses]
A metabolite of Budesonide (B689490), an antiinflammatory agent. | [Uses]
A metabolite of Budesonide, an antiinflammatory agent | [Synthesis]
501 g of crude 16α-hydroxy prednisolone acetate is dissolved in 700 ml of dichloromethane and 700 ml of ethanol, then 1.5 ml of formic acid is added, 24 ml of 5% sodium hypochlorite aqueous solution is added dropwise, and the temperature is controlled at 15 °C, and the reaction is stirred to obtain the preliminary processed product; Add 150 ml of 5% sodium bisulfite aqueous solution to the above-mentioned preliminary treatment product, concentrate on removing the solvent, adding water to hydrolyze, and filter to obtain 45 g of 16α-hydroxyprednisolone acetate refined product; 45 g of 16α-hydroxyprednisolone acetate obtained above was dissolved in a mixed organic solvent of 250 ml of dichloromethane and 250 ml of methanol, stirred and cooled to 0 °C, 90 ml of 10% sodium sulfite aqueous solution was added, and the reaction was stirred. After completion, it was neutralized with 15% sulfuric acid by mass concentration; the mixed organic solvent was concentrated, watered out, filtered, and dried to obtain 36.5 g of 16α-hydroxyprednisolone (11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione).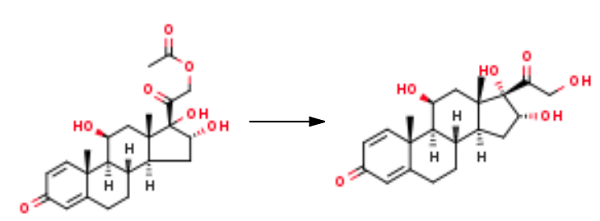 |
|
|