| Identification | More | [Name]
Titanium dioxide | [CAS]
13463-67-7 | [Synonyms]
ANATASE
FERRISPEC(R) PL TITANIUM DIOXIDE WHITE
HOMBIKAT
PIGMENT WHITE 6
RUTILE
TIO2
TITAN DIOXIDE
TITANIA
TITANIC ANHYDRIDE
TITANIUM(+4)OXIDE
TITANIUM DIOXIDE
TITANIUM DIOXIDE, ANATASE
TITANIUM DIOXIDE, RUTILE
TITANIUM DIOXIDE, RUTILE FORM
TITANIUM DIOXIDE RUTILE TITAN (TM) R-02
TITANIUM DIOXIDE RUTILE TYTANPOL(TM)
TITANIUM(IV) DIOXIDE
TITANIUM(IV) OXIDE
TITANIUM(IV) OXIDE, ANATASE FORM
TITANIUM(IV) OXIDE, RUTILE | [EINECS(EC#)]
215-280-1 | [Molecular Formula]
O2Ti | [MDL Number]
MFCD00011269 | [Molecular Weight]
79.87 | [MOL File]
13463-67-7.mol |
| Chemical Properties | Back Directory | [Appearance]
Ttitanium dioxide is an odorless white powder. | [Melting point ]
1840 °C
| [Boiling point ]
2900 °C
| [bulk density]
850kg/m3 | [density ]
4.26 g/mL at 25 °C(lit.)
| [refractive index ]
2.61 | [Fp ]
2500-3000°C | [storage temp. ]
Store at +5°C to +30°C. | [solubility ]
Practically insoluble in water. It does not dissolve in dilute mineral acids but dissolves slowly in hot concentrated sulfuric acid. | [form ]
powder
| [color ]
White to slightly yellow | [Specific Gravity]
4.26 | [Odor]
at 100.00?%. odorless | [PH]
7-8 (100g/l, H2O, 20℃)(slurry) | [Water Solubility ]
insoluble | [Crystal Structure]
Orthorhombic, Pcab | [Merck ]
14,9472 | [Dielectric constant]
2.9(20℃) | [Exposure limits]
ACGIH: TWA 10 mg/m3
OSHA: TWA 15 mg/m3
NIOSH: IDLH 5000 mg/m3; TWA 2.4 mg/m3; TWA 0.3 mg/m3 | [Uses]
titanium dioxide (TiO2) is one of the 21 FDA-approved sunscreen chemicals with an approved usage level of 2 to 25 percent. When applied, titanium dioxide remains on the skin’s surface, scattering uV light. It is often used in conjunction with other sunscreen chemicals to boost the product’s SPF value, thus reducing the risk of irritation or allergies attributed to excessive usage of chemical sunscreens. Its incorporation into sunscreen formulations, makeup bases, and daytime moisturizers depends on the particular size of titanium dioxide employed. The smaller the particle size, the more unobtrusive Tio2’s application. Large particles, on the other hand, leave a whitish wash or look on the skin. Some companies list “micro” or “ultra” when referring to the size of the titanium dioxide particle. According to some sources, titanium dioxide could be the ideal uVA/uVB protection component given its chemical, cosmetic, and physical characteristics. Titanium dioxide is also used to provide a white color to cosmetic preparations. | [CAS DataBase Reference]
13463-67-7(CAS DataBase Reference) | [IARC]
2B (Vol. 47, 93) 2010 | [NIST Chemistry Reference]
Titanium dioxide(13463-67-7) | [EPA Substance Registry System]
13463-67-7(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
| [Risk Statements ]
R20:Harmful by inhalation.
R36/37/38:Irritating to eyes, respiratory system and skin .
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R38:Irritating to the skin.
R20/21:Harmful by inhalation and in contact with skin .
R10:Flammable.
R36/38:Irritating to eyes and skin .
R22:Harmful if swallowed. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S25:Avoid contact with eyes .
S2:Keep out of the reach of children .
S36/37:Wear suitable protective clothing and gloves . | [WGK Germany ]
-
| [HS Code ]
28230000 | [Safety Profile]
A nuisance dust. A
human skin irritant. Questionable
carcinogen with experimental carcinogenic,
neoplastigenic, and tumorigenic data.
Violent or incandescent reaction with metals
at high temperatures (e.g., aluminum,
calcium, magnesium, potassium, sodium,
zinc, lithium). See also TITANIUM
COMPOUNDS. | [Hazardous Substances Data]
13463-67-7(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: > 10000 mg/kg | [IDLA]
5,000 mg/m3 |
| Hazard Information | Back Directory | [Potential Exposure]
Titanium dioxide is a white pigment used as a pigment in paint; in the rubber, plastics, ceramics, paint, and varnish industries, in dermatological preparations; and is used as a starting material for other titanium compounds; as a gem; in curing concrete; and in coatings for welding rods. It is also used in paper and cardboard manufacture. | [First aid]
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 minutes, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. | [Incompatibilities]
Titanium dioxide is incompatible with strong oxidizers and strong acids. Violent or incandescent reactions may occur with metals (e.g., aluminum, calcium, magnesium, potassium, sodium, zinc, and lithium). | [Description]
Titanium dioxide, TiO2, is a white powder and has the greatest hiding power of all white pigments. It is noncombustible; however, it is a powder and, when suspended in air, may cause a dust explosion if an ignition source is present. It is not listed in the DOT Hazardous Materials Table, and the DOT does not consider it hazardous in transportation. The primary uses are as a white pigment in paints, paper, rubber, and plastics; in cosmetics; in welding rods; and in radioactive decontamination of the skin. | [Waste Disposal]
Land fill. | [Physical properties]
The naturally occurring dioxide exists in three crystal forms: anatase, rutile and brookite. While rutile, the most common form, has an octahedral structure. Anatase and brookite have very distorted octahedra of oxygen atoms surrounding each titanium atom. In such distorted octahedral structures, two oxygen atoms are relatively closer to titanium than the other four oxygen atoms. Anatase is more stable than the rutile form by about 8 to 12 kJ/mol (Cotton, F.A., Wilkinson, G., Murillo, C.A and M Bochmann. 1999. Advanced Inorganic Chemistry, 6th ed, p. 697, New York: John Wiley & Sons) Other physical properties are: density 4.23g/cm3; Mohs hardness 5.8 g/cm3 ( anatase and brookite) and 6.2 g/cm3 ( rutile); index of refraction 2.488 (anatase), 2.583 (brookite) and 2.609 (rutile); melts at 1,843°C; insoluble in water and dilute acids; soluble in concentrated acids. | [Occurrence]
Titanium dioxide occurs in nature in the crystalline forms rutile, anatase,
and brookite. Rutile and anatase are manufactured in large quantities, which are
primarily used as pigments, but also as catalysts and in ceramics. | [Characteristics]
Titanium dioxide (C.I. Pigment White 6) is of outstanding importance as a white pigment because of its scattering properties, its chemical stability, its biological inertness, and its lack of toxicity. The pigment is frequently coated with colorless organic or inorganic compounds of low solubility to improve its weather resistance, lightfastness, and dispersibility.
| [Definition]
ChEBI: Titanium dioxide is a titanium oxide with the formula TiO2. A naturally occurring oxide sourced from ilmenite, rutile and anatase, it has a wide range of applications. It has a role as a food colouring. | [Production Methods]
There are two major processes for the manufacture of titanium dioxide
pigments, namely sulfate route and chloride route. In the sulfate
process, the ore limonite, FeOTiO2, is dissolved in sulfuric acid and
the resultant solution is hydrolyzed by boiling to produce a hydrated
oxide, while the iron remains in solution. The precipitated titanium
hydrate is washed and leached free of soluble impurities. Controlled calcinations
at about 1000°C produce pigmentary titanium dioxide of the
correct crystal size distribution; this material is then subjected to a finishing
coating treatment and milling.
The chloride process uses gaseous chlorination of mineral rutile, followed
by distillation and finally a vapor phase oxidation of the titanium
tetrachloride. | [Production Methods]
Titanium dioxide occurs naturally as the minerals rutile (tetragonal
structure), anatase (tetragonal structure), and brookite (orthorhombic
structure).
Titanium dioxide may be prepared commercially by either the
sulfate or chloride process. In the sulfate process a titanium
containing ore, such as ilemenite, is digested in sulfuric acid. This
step is followed by dissolving the sulfates in water, then precipitating
the hydrous titanium dioxide using hydrolysis. Finally, the
product is calcinated at high temperature. In the chloride process,
the dry ore is chlorinated at high temperature to form titanium
tetrachloride, which is subsequently oxidized to form titanium
dioxide. | [Composition]
This material is visually a brilliant white pigment which also has anti-inflammatory properties. Two crystal types of TiO2 occur: anatase and rutile. In order to produce these crystals, there are two manu�facturing processes that are employed: (1) The sulfate manufacturing process has the ability to produce either type of crystal, while (2) the chloride manufacturing process produces only rutile crystals. | [General Description]
Two main physico-chemically distinct polymorphs of TiO2 are anatase and rutile. Anatase has a higher photocatalytic activity than rutile but is thermodynamically less stable. | [Hazard]
Lower respiratory tract irritant. Possible
carcinogen. | [Health Hazard]
Titanium dioxide is a mild pulmonary
irritant and is generally regarded as a
nuisance dust. | [Flammability and Explosibility]
Notclassified | [reaction suitability]
reagent type: catalyst
core: titanium | [Pharmaceutical Applications]
Titanium dioxide is widely used in confectionery, cosmetics, and
foods, in the plastics industry, and in topical and oral pharmaceutical
formulations as a white pigment.
Owing to its high refractive index, titanium dioxide has lightscattering
properties that may be exploited in its use as a white
pigment and opacifier. The range of light that is scattered can be
altered by varying the particle size of the titanium dioxide powder.
For example, titanium dioxide with an average particle size of
230nm scatters visible light, while titanium dioxide with an average particle size of 60nm scatters ultraviolet light and reflects visible
light.
In pharmaceutical formulations, titanium dioxide is used as a
white pigment in film-coating suspensions, sugar-coated tablets,
and gelatin capsules. Titanium dioxide may also be admixed with
other pigments.
Titanium dioxide is also used in dermatological preparations
and cosmetics, such as sunscreens. | [Safety]
Titanium dioxide is widely used in foods and oral and topical
pharmaceutical formulations. It is generally regarded as an
essentially nonirritant and nontoxic excipient. | [Carcinogenicity]
Carcinogenesis. In a 1985 study, rats (CD) were
exposed to graded airborne concentrations (0, 10, 50, and
250mg/m3) of TiO2 6 h/day, 5 days/week, for 2 years. The
majority of the particles were in the respirable range (84%
≤13 mmMMD). All responses were confined to the lungs. At
the lowest dose, the histopathological evaluation of the lungs
revealed dust-laden macrophages in the alveolar ducts and
adjacent alveoli with pneumocyte hyperplasia. At the two
highest concentrations, there were increases in lung weight,
accumulation of dust in the macrophages, foamy macrophage
responses, type II pneumocyte hyperplasia, alveolar proteinosis,
alveolar bronchiolization, cholesterol granulomas, focal
pleurisy, and dust deposition in the tracheobronchiolar lymph
nodes. At the 250mg/m3 exposure concentration, bronchiole
alveolar adenomas (males: control 2/79, 250mg/m3 12/79;
females: control 0/79, 250mg/m3 13/79) increased.
Additionally, 13/79 females at the 250mg/m3 dose showed squamous cell carcinoma, compared with none in 79 controls.
Theauthorsnoted that this responsemight have little biological
relevance to humans because of the overload of respiratory
clearance mechanisms and also pointed out that the type,
location, and development of the tumors were different from
those in human lung tumors. It is not clear that the nasal
cavity epithelium was examined. However, the nasal cavity
load would be expected to be higher in the rats because of
anatomic structure, whereas the lung deposition should be
higher in humans because we are, in part, mouth breathers. | [storage]
Titanium dioxide is extremely stable at high temperatures. This is
due to the strong bond between the tetravalent titanium ion and the
bivalent oxygen ions. However, titanium dioxide can lose small,
unweighable amounts of oxygen by interaction with radiant energy.
This oxygen can easily recombine again as a part of a reversible
photochemical reaction, particularly if there is no oxidizable
material available. These small oxygen losses are important because
they can cause significant changes in the optical and electrical
properties of the pigment.
Titanium dioxide should be stored in a well-closed container,
protected from light, in a cool, dry place. | [Forms and nomenclature]
Titanium dioxide occurs in nature in three polymorphic crystal forms: anatase, rutile, and brookite.
Moreover, under high pressure, the structure of all three polymorphs of titanium dioxide
may be converted into that of α-PbO2. The following diagram summarises the main properties of these three polymorphisms:
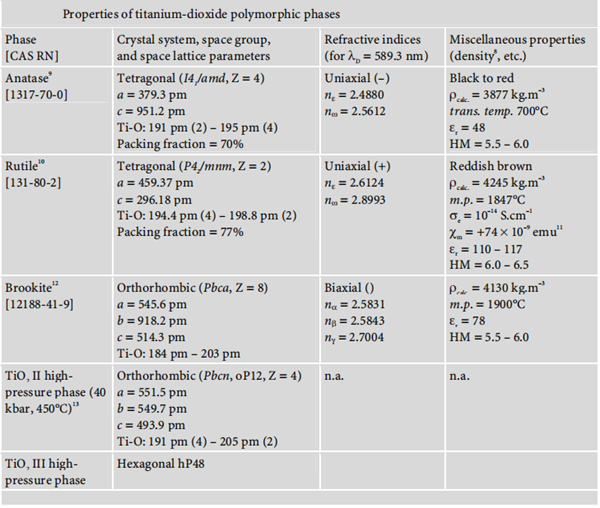 | [Structure and conformation]
Rutile and anastase crystals are tetragonal. Rutile crystals have greater coverage due to the close packing orientation of the atoms in the crystal. The refractive indices for anatase and rutile crystals are 2.55 and 2.71, respectively. The resultant opacity is due to the light scattering ability of the TiO2. Light, heat, and chemical stability are excellent when employing this material. Additionally, in the United States, TiO2 is regarded as a Category I sunscreen. | [Toxics Screening Level]
The Initial Threshold Screening Level (ITSL) for titanium dioxide is 24 μg/m3 with an 8-hour
averaging time. | [Regulatory Status]
Accepted as a food additive in Europe. Included in the FDA Inactive
Ingredients Database (dental paste; intrauterine suppositories; ophthalmic preparations; oral capsules, suspensions, tablets; topical
and transdermal preparations). Included in nonparenteral medicines
licensed in the UK. Included in the Canadian List of
Acceptable Non-medicinal Ingredients. |
| Questions and Answers (Q&A) | Back Directory | [Chemical Properties]
The naturally occurring dioxide exists in three crystal forms: anatase, rutile and brookite. While rutile, the most common form, has an octahedral structure. Anatase and brookite have very distorted octahedra of oxygen atoms surrounding each titanium atom. In such distorted octahedral structures, two oxygen atoms are relatively closer to titanium than the other four oxygen atoms. Anatase is more stable than the rutile form by about 8 to 12 kJ/mol (Cotton, F.A., Wilkinson, G., Murillo, C.A and M Bochmann. 1999. Advanced Inorganic Chemistry, 6th ed, p. 697, New York: John Wiley & Sons) Other physical properties are: density 4.23g/cm3; Mohs hardness 5.8 g/cm3 ( anatase and brookite) and 6.2 g/cm3 ( rutile); index of refraction 2.488 (anatase), 2.583 (brookite) and 2.609 (rutile); melts at 1,843°C; insoluble in water and dilute acids; soluble in concentrated acids.
| [Application]
Industry
Application
Role/benefit
Pigment
Optical coating for dielectric mirrors and gemstones
Brightness and very high refractive index
Paper coating
Helps to make paper whiter, brighter and more opaque
Plastics, adhesives and rubber
Helps minimize the brittleness, fading and cracking that can occur as a result of light exposure
Food Contact materials and ingredients
Prevents premature degradation and enhance the longevity of the product
Paints
Gives paint its high gloss and rich depth of color
Ceramic glazes
Acts as an opacifier and seeds crystal formation
Cosmetic
Sunscreens
Active ingredients/high refractive index and strong UV light absorbing capabilities
Daily cosmetics or make-up materials
Additive/aids in hiding blemishes and brightening the skin
Toothpastes
Additive/helps to whiten tooth
Catalyst
Dye-sensitized solar cell
Can produce electricity in nanoparticle form
Hydrolysis reaction
Catalyzes the photo decomposition of water into hydrogen and oxygen
Automotive, power stations, etc.
Helps to removes harmful exhaust gas emissions, such as nitrous oxides, volatile organic compounds, etc.
Detoxification or remediation of wastewater
Photocatalytically mineralizes pollutants (to convert into CO2 and H2O) in waste water
Photocatalytic antimicrobial coating
Photocatalytic destruction of organic matter
Others
Oxygen sensor
The electrical resistivity of TiO2 can be correlated to the oxygen content of the atmosphere
Anti-fogging coatings and self-cleaning windows
Under exposure to UV light, TiO2 becomes increasingly hydrophilic
Coated ceramic tile
Disinfectant and self-cleaning qualities
Treatment of the air in fruit, vegetable and cut flower storage areas
Removes ethylene gas to prevent spoilage and prevents internal combustion
Memristor
Can be employed for solar energy conversion
Mixed conductor
Significant ionic and electronic conduction
| [Uses]
Titanium dioxide is an extreme white and bright compound with high index of refraction. In paints it is a white pigment and an opacifying agent.It is in house paints, water paints, lacquers, enamels, paper filling and coating, rubber, plastics, printing ink, synthetic fabrics, floor coverings, and shoe whiteners. Also, it is used in colorants for ceramics and coatings for welding rods. A rutile form of the dioxide is used in synthetic gem stones.
| [Preparation]
Titanium dioxide is mined from natural deposits. It also is produced from other titanium minerals or prepared in the laboratory. Pigment-grade dioxide is produced from the minerals, rutile and ilmenite. Rutile is converted to pigment grade rutile by chlorination to give titanium tetrachloride, TiCl4. Anhydrous tetrachloride is converted back to purified rutile form by vapor phase oxidation.
Anatase form is obtained by hydrolytic precipitation of titanium(IV) sulfate on heating. The mineral ilmenite is treated with concentrated sulfuric acid. Heating the sulfate solution precipitates hydrous titanium oxide. The precipitate is calcined to expel all water.
Titanium dioxide also can be prepared by heating Ti metal in air or oxygen at elevated temperatures.
|
| Questions And Answer | Back Directory | [Uses]
Titanium (IV) dioxide (TiO2), also known as rutile, is one of the best-known compounds used as a paint pigment. Of the 3.06 million metric tons of Ti02 used in 1992, 51 % was used in coatings, 19% in plastics, 14% in paper, and the balance of 8% in several different applications such as elastomers, ceramics, cosmetics, and foods. It is ideal for paints exposed to severe temperatures and marine climates because of its inertness and self-cleaning attributes. It is also used in manufacture of glassware, ceramics, enamels, welding rods, and floor coverings. |
|
|