| Identification | Back Directory | [Name]
DICHLORO[(R)-(+)-2,2'-BIS(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHYL]RUTHENIUM (II) | [CAS]
134524-84-8 | [Synonyms]
Dichloro [(S)
(S)-[2,2′
(S)-BINAP RUCL2
-binaphthyl]dichlororuthenium
-1,1'-binaphthyl]ruthenium(II)
-binaphthyl]ruthenium(II),(S-BINAP)RuCl2
Dichlorobisdiphenylphosphinobinaphthylrutheniu
(S)-(-)2,2'bis(diphenylphosphino)-1,1'-binaphthyl]ruthenium(II)
(S)-[2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl]dichlororuthenium
(S)-[2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl]dichlororuthenium 95%
Dichloro〔(S)-(-)-2,2-bis(diphenylphosphino)-1,1-binaphthyl〕ruthenium(Ⅱ)
[(S)-2,2'-Bis(diphenylphosphino)-1,1'-binaphthyl]ruthenium(II) Dichloride
(S)-(-)-2,2''-Bis-(diphenylphosphino)-1,1''-binaphthyl ruthenium dichloride
DICHLORO[(R)-(+)-2,2'-BIS(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHYL]RUTHENIUM (II)
[(1S)-[1,1′-Binaphthalene]-2,2′-diylbis[diphenylphosphine-κP]]dichloro-ruthenium
Dichloro[(S)-(-)-2,2'-bis(diphenylphosphino)-1,1'-binaphthyl]ruthenium(II),min.95%
DICHLORO[(R)-2,2'-BIS-(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHYL]RUTHENIUM(II) HOMOPOLYMER
DICHLORO[(S)-2,2'-BIS-(DIPHENYLPHOSPHINO)-1,1'-BINAPHTHYL]RUTHENIUM(II) HOMOPOLYMER
Dichloro[(S)-(-)-2,2'-bis(diphenylphosphino)-1,1'-binaphthyl]ruthenium(II), min. 95%
Dichloro[(S)-(+)-2,2'-bis(diphenylphosphino)-1,1'-binaphthyl]rutheniuM(II),(S-BINAP)RuCl2
Dichloro[(S)-(+)-2,2'-bis(diphenylphosphino)-1,1'-binaphthyl]rutheniuM(II),95% [(S)-BINAP]RuCl2 | [EINECS(EC#)]
1312995-182-4 | [Molecular Formula]
C44H32Cl2P2Ru | [MDL Number]
MFCD01073794 | [MOL File]
134524-84-8.mol | [Molecular Weight]
794.65 |
| Chemical Properties | Back Directory | [Melting point ]
>300 ºC | [storage temp. ]
Inert atmosphere,2-8°C | [solubility ]
Benzene (Slightly, Heated), Dichloromethane (Slightly, Heated) | [form ]
Powder | [color ]
orange | [Sensitive ]
air sensitive | [Stability:]
Air Sensitive, Moisture Sensitive |
| Hazard Information | Back Directory | [Chemical Properties]
Solid | [Uses]
[(S)-2,2''-Bis(diphenylphosphino)-1,1''-binaphthyl]dichlororuthenium is used as a catalyst in the asymmetric synthesis of chiral δ-lactones and in the stereoselective synthesis of (hydroxy)amino acids via asymmetric hydrogenation of aminoketo esters. |
| Questions And Answer | Back Directory | [Reaction]
- Enantioselective catalyst for the asymmetric hydrogenation of α,β-unsaturated olefins.
- Efficient catalyst for the asymmetric reduction of carbonyl groups, such as β-ketoesters.
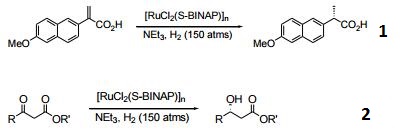
|
|
|