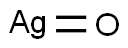| Identification | More | [Name]
Silver oxide | [CAS]
1301-96-8 | [Synonyms]
SILVER (II) OXIDE
SILVER OXIDE
silveroxide(ago)
Silver(II) oxide, 99.9%
SILVER(II) OXIDE (AgO)
Argentic oxide
Silver suboxide
Silver(II) oxide, 85.5-88.0% Ag
SILVER (II) OXIDE TECHNICAL
Silver(II)oxide(99.9%-Ag)
SILVER(II) OXIDE (87%AG)
Silver oxide
Silver(II) oxide, 99.9% (metals basis), Ag 86.6% min
Divasil
Silver monoxide | [EINECS(EC#)]
215-098-2 | [Molecular Formula]
Ag2O | [MDL Number]
MFCD00044285 | [Molecular Weight]
231.74 | [MOL File]
1301-96-8.mol |
| Safety Data | Back Directory | [Hazard Codes ]
O,Xi,C | [Risk Statements ]
R8:Contact with combustible material may cause fire.
R36/37/38:Irritating to eyes, respiratory system and skin .
R34:Causes burns. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S17:Keep away from combustible material . | [RIDADR ]
UN 1479 5.1/PG 2
| [WGK Germany ]
3
| [TSCA ]
Yes | [HazardClass ]
5.1 | [PackingGroup ]
II | [HS Code ]
28432900 |
| Questions And Answer | Back Directory | [Physical Properties]
Gray monoclinic or cubic crystals or powder; diamagnetic; semiconductor; density 7.48 g/cm3; decomposes to its elements above 100°C; insoluble in water (solubility 27 mg/L at 25°C); soluble in alkalis; decomposes in ammonia solution evolving nitrogen; dissolves in dilute acids with decomposition evolving oxygen; forms a brown solution in concentrated nitric acid, and forms intense green coloration in concentrated sulfuric acid.
| [Uses]
Silver(II) oxide is used to make silver oxide-zinc alkali batteries. Also, it is an oxidizing agent.
| [Preparation]
Silver(II) oxide is prepared by reacting silver nitrate with potassium persulfate in the presence of a base.
|
| Hazard Information | Back Directory | [Chemical Properties]
grey to black powder | [Purification Methods]
Silver (II) oxide [1301-96-8] M 123.9, m > 1 0 0o(dec), 7.22. It is soluble in 40,000 parts of H2O, and should be protected from light. Stir it with an alkaline solution of potassium peroxysulfate (K2S2O8) at 85-90o. The black AgO is collected, washed free from sulfate with H2O made slightly alkaline and dried in air in the dark. [Hammer & Kleinberg Inorg Synth IV 12 1953.] |
|
| Company Name: |
Alfa Aesar
|
| Tel: |
400-6106006 |
| Website: |
http://chemicals.thermofisher.cn |
|