| Identification | More | [Name]
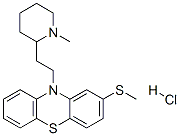
Thioridazine hydrochloride | [CAS]
130-61-0 | [Synonyms]
10-[2-(1-METHYL-2-PIPERIDYL)ETHYL]-2-(METHYLTHIO)-10H-PHENOTHIAZINE HYDROCHLORIDE
10-[2-(1-METHYL-2-PIPERIDYL)-ETHYL]-2-[METHYLTHIOL]-PHENOTHIAZINE HYDROCHLORIDE
LABOTEST-BB LT00451961
THIORIDAZINE HCL
10-(2-(1-methyl-2-piperidinyl)ethyl)-2-(methylthio)-10h-phenothiazinmono
10-(2-(1-methyl-2-piperidyl)ethyl)-2-(methylthio)-phenothiazinmonohydrochl
10-(2-(1-methyl-2-piperidyl)ethyl)-2-methylthiophenothiazinehydrochloride
2-methylmercapto-10-(2-(n-methyl-2-piperidyl)ethyl)phenothiazinehydrochlorid
mellarilhydrochloride
melleril(tablet)
usafsz-3
ThioridazinHCl
Thioridazinhydrochlorid
10-[2-(1-Methyl-2-piperidinyl)ethyl]-2-(methylthio)-10H-phenothiazine Hydrochloride
2-Methylmercapto-10-[2-(N-methyl-2-piperidyl)ethyl]phenothiazine Hydrochloride
Orsanil
Ridazin
Stalleril
10H-Phenothiazine, 10-2-(1-methyl-2-piperidinyl)ethyl-2-(methylthio)-, monohydrochloride
THIORIDAZINEHYDROCHLORIDE,USP | [EINECS(EC#)]
204-992-8 | [Molecular Formula]
C21H27ClN2S2 | [MDL Number]
MFCD00012655 | [Molecular Weight]
407.04 | [MOL File]
130-61-0.mol |
| Chemical Properties | Back Directory | [Appearance]
Pale Yellow Solid | [Melting point ]
158-1600C | [Boiling point ]
230°C (rough estimate) | [density ]
1.1227 (rough estimate) | [refractive index ]
1.5610 (estimate) | [storage temp. ]
2-8°C | [solubility ]
H2O: soluble250 mg plus 5 ml of solvent, clear, colorless to faintly yellow | [form ]
Off-white solid | [color ]
White to Almost white | [Water Solubility ]
Soluble in water (75 mM), DMSO (100 mM), chloroform, ethanol, and methanol. | [Sensitive ]
Light Sensitive & Hygroscopic | [Usage]
Dopamine receptor blocker; parent compound of sulforidazine and mesoridazine. Antipsychotic | [λmax]
317nm(EtOH)(lit.) | [Merck ]
13,9435 | [InChIKey]
NZFNXWQNBYZDAQ-UHFFFAOYSA-N | [CAS DataBase Reference]
130-61-0(CAS DataBase Reference) | [NIST Chemistry Reference]
Thioridazine hydrochloride(130-61-0) | [EPA Substance Registry System]
130-61-0(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,N | [Risk Statements ]
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin .
R50/53:Very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36:Wear suitable protective clothing .
S60:This material and/or its container must be disposed of as hazardous waste .
S61:Avoid release to the environment. Refer to special instructions safety data sheet . | [RIDADR ]
UN 3077 9/PG 3 | [WGK Germany ]
3 | [RTECS ]
SP2275000 | [HazardClass ]
9 | [HS Code ]
2934302300 |
| Hazard Information | Back Directory | [Chemical Properties]
Pale Yellow Solid | [Originator]
Mellaril,Sandoz,US,1959 | [Uses]
Thioridazine hydrochloride has been used as an intercalating agent for analyzing the integrity of double-stranded DNA (dsDNA) using square-wave voltammetry (SWV) techniques. Thioridazine hydrochloride has also been used as a positive control for the inhibition of hepatic enzyme cytochrome P4502D6 (CYP2D6) in human liver microsomes. | [Uses]
Dopamine receptor blocker; parent compound of sulforidazine and mesoridazine. Antipsychotic | [Uses]
Thioridazine HCl is a dopamine receptor blocker and antipsychotic. It is the parent compound of sulforidazine and mesoridazine.
This compound has been reported to bind strongly to dopamine receptors on cancer stem cells and cause differentiation leaving normal cells alone (see Can. Chem. News 12 July/August 2012). | [Definition]
ChEBI: Thioridazine hydrochloride is a hydrochloride. It has a role as a first generation antipsychotic and a geroprotector. It contains a thioridazine. | [Manufacturing Process]
N-(m-methylmercapto-phenyl)-aniline (MP 59° to 61°C) is prepared by
condensing m-methylmercapto-aniline (BP 163° to 165°C/16 mm Hg) with the
potassium salt of o-chloro-benzoic acid and decarboxylating the resultant N-
(m-methylmercapto-phenyl)-anthranilic acid (MP 139° to 141°C) by heating,
and then distilling.
9.87 parts of N-(m-methylmercapto-phenyl)-aniline are heated with 2.93
parts of sulfur and 0.15 part of powdered iodine for 15 minutes in a bath at
about 160°C. Upon termination of the ensuing evolution of hydrogen sulfide,
animal charcoal is added to the reaction mixture and recrystallization carried
out first from 40 parts by volume of chlorobenzene, and then from 25 to 30
parts by volume of benzene at the boiling temperature. The obtained citronyellow
3-methylmercapto-phenothiazine has a MP of 138° to 140°C.
17.82 parts of 2-methylmercapto-phenothiazine, 3.4 parts of finely pulverized sodamide and 80 parts by volume of absolute xylene are heated to boiling for
two hours at a bath temperature of 180°C under a reflux condenser and while
stirring the reaction mixture. Without interrupting the heating, a solution of
13.2 parts of 2-(N-methyl-piperidyl-2')-1chloro-ethane in 40 parts by volume
of absolute xylene is then added dropwise in the course of 1 1/2 hours. After
further heating for 3 hours, the reaction mixture is cooled and, after the
addition of 5 parts of ammonium chloride, is shaken three times with water,
using 25 parts by volume each time. The xylene solution is extracted once
with 35 parts by volume of 3 normal acetic acid and then three times, each
time with 15 parts by volume of the said acid, after which the acetic acid
extract is washed with 60 parts by volume of ether and is then made
phenolphthalein-alkaline by means of 25 parts by volume of concentrated
aqueous caustic soda solution.
The precipitated oily base is taken up in a total of 100 parts by volume of
benzene. The benzene layer, dried over potassium carbonate, is filtered and
then evaporated under reduced pressure. The residue from the evaporation is
distilled in a high vacuum; after separating a preliminary distillate which
passes over up to 228°C under a pressure of 0.92 mm Hg, the principal
fraction, 2-methylmercapto-10-[2'-(N-methyl-piperidyl-2'')-ethyl-
1']phenothiazine, which distills over at 228° to 232°C under the lastmentioned
pressure, is collected. The analytically pure base has a BP of
230°C/0.02 mm Hg. | [Therapeutic Function]
Tranquilizer | [General Description]
Thioridazine hydrochloride,10-[2-(1-methyl-2-piperidyl)ethyl]-2-(methylthio)phenothiazine monohydrochloride (Mellaril), is amember of the piperidine subgroup of the phenothiazines.The drug has a relatively low tendency to produce EPS. Thedrug has high anticholinergic activity, and this activity in thestriatum, counterbalancing a striatal DA block, may be responsiblefor the low EPS. It also has been suggested thatthere may be increased DA receptor selectivity, which may be responsible. The drug has sedative and hypotensive activityin common with chlorpromazine and less antiemeticactivity. At high doses, pigmentary retinopathy has been observed.Its major metabolites include N-demethylated, ringhydroxylated,and S-oxidized products. Thioridazine isprominently converted to the active metabolite mesoridazine(discussed next), which probably contributes to the antipsychoticactivity of thioridazine. | [Biological Activity]
Dopamine receptor antagonist that displays antipsychotic activity. | [Biochem/physiol Actions]
D2 dopamine receptor antagonist; phenothazine antipsychotic with reduced extrapyramidal side effects; Ca2+ channel blocker. | [storage]
Desiccate at RT |
|
|