| Identification | Back Directory | [Name]
N-[3-[5-(2-Amino-4-pyrimidinyl)-2-(tert-butyl)-4-thiazolyl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide | [CAS]
1195765-45-7 | [Synonyms]
Dabrafenib
GSK2118436A
Debrafenib API
Dabrafenib Base
Dabrafenib, >=98%
Dabrafenib
KB-57246
Debrafenib free base
Dabrafenib(free base)
Dabrafenib (GSK2118436)
Dabrafenib (GSK2118436A)
Dabrafenib(GSK2118436)
KB-57246
Dabrafenib free base(GSK2118436A)
GSK2118436, Dabrafenib, GSK2118436A
N-(3-(5-(2-Aminopyrimidin-4-yl)-2-tert-butylthiazol-4-yl)-2-fluorophenyl)-2,6-difluorobenzenes
N-(3-(5-(2-aminopyrimidin-4-yl)-2-tert-butylthiazol-4-yl)-2-fluorophenyl)-2,6-difluorobenzenesulfonamide
N-[3-[5-(2-Amino-4-pyrimidinyl)-2-(tert-butyl)-4-thiazolyl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide
BenzenesulfonaMide, N-[3-[5-(2-aMino-4-pyriMidinyl)-2-(1,1-diMethylethyl)-4-thiazolyl]-2-fluorophenyl]-2,6-difluoro-
N-[3-[5-(2-Amino-4-pyrimidinyl)-2-(tert-butyl)-4-thiazolyl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide Dabrafenib
Dabrafenib N-[3-[5-(2-Amino-4-pyrimidinyl)-2-(tert-butyl)-4-thiazolyl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide | [EINECS(EC#)]
689-166-9 | [Molecular Formula]
C23H20F3N5O2S2 | [MDL Number]
MFCD17215684 | [MOL File]
1195765-45-7.mol | [Molecular Weight]
519.562 |
| Chemical Properties | Back Directory | [Melting point ]
214-216oC | [Boiling point ]
653.7±65.0 °C(Predicted) | [density ]
1.443 | [storage temp. ]
-20°C | [solubility ]
Soluble in DMSO (up to 30 mg/ml with warming), or in Ethanol (up to 1 mg/ml with warming). | [form ]
White solid. | [pka]
6.62±0.10(Predicted) | [color ]
Off-white | [Stability:]
Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. | [InChIKey]
BFSMGDJOXZAERB-UHFFFAOYSA-N | [SMILES]
C1(S(NC2=CC=CC(C3=C(C4C=CN=C(N)N=4)SC(C(C)(C)C)=N3)=C2F)(=O)=O)=C(F)C=CC=C1F |
| Hazard Information | Back Directory | [Definition]
ChEBI: An organofluorine compound and antineoplastic agent, used as its mesylate salt in treatment of metastatic melanoma. | [Originator]
GlaxoSmithKline (United States) | [Uses]
Dabrafenib is an inhibitor of mutated BRAF kinase and has clinical activity with a manageable safety profile in clinical trials of phase 1 and 2 in patients with BRAF(V600)-mutated metastatic melanoma. | [Brand name]
Tafinlar | [General Description]
Class: dual threonine/tyrosine kinase;
Treatment: melanoma with BRAF mutations; Oral bioavailability = 95%;
Elimination half-life = 8 h;
Protein binding = 99.7% | [Pharmacokinetics]
Dabrafenib exhibits an oral bioavailability of
95%, indicative of extensive absorption and low firstpass intestinal and hepatic metabolism. The
excellent oral bioavailability contributes to a much
lower dosage than vemurafenib (150 mg, BID vs.
960 mg, BID). It has an elimination half-life of 8 h,
resulting in twice-daily dosing regimen. Dabrafenib undergoes metabolism primarily via
oxidation of the t-butyl group to form hydroxydabrafenib 6, which is further oxidized to carboxydabrafenib 7. Subsequent decarboxylation furnishes
the desmethyl-dabrafenib 8 via a pH-dependent
decarboxylation (Fig. 4). The major route of
elimination of dabrafenib is a combination of
oxidative metabolism (48% of the dose) and biliary
excretion.
 | [Clinical Use]
Selective inhibitor of BRAF-kinase:
Treatment of metastatic melanoma and advanced
non-small cell lung cancer with a BRAF V600
mutation | [target]
B-Raf (V600E) | [Drug interactions]
Potentially hazardous interactions with other drugs
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis.
Oestrogens and progestogens: possibly reduced
contraceptive effect. | [Metabolism]
Metabolism is mainly by CYP2C8 and CYP3A4
isoenzymes to form hydroxy-dabrafenib, which is further
oxidised via CYP3A4 to form carboxy-dabrafenib.
Carboxy-dabrafenib can be decarboxylated via a non�enzymatic process to form desmethyl-dabrafenib.
Carboxy-dabrafenib is excreted in bile and urine.
Desmethyl-dabrafenib may also be formed in the gut
and reabsorbed. Desmethyl-dabrafenib is metabolised
by CYP3A4 to oxidative metabolites. Both hydroxy�and desmethyl-dabrafenib are likely to contribute to
the clinical activity of dabrafenib while the activity of
carboxy-darafenib is not likely to be significant. | [storage]
Store at -20°C |
| Questions And Answer | Back Directory | [Description]
N-[3-[5-(2-Amino-4-pyrimidinyl)-2-(tert-butyl)-4-thiazolyl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide is well known as dabrafenib. It is a drug for the treatment of cancers associated with a mutated version of the gene BRAF. Dabrafenib acts as an inhibitor of the associated enzyme B-Raf, which plays a role in the regulation of cell growth. Dabrafenib has clinical activity with a manageable safety profile in clinical trials of phase-I and -II in patients with BRAF(V600)-mutated metastatic melanoma1,2. Its mechanism of action is acted as a Protein Kinase Inhibitor, and Cytochrome P450 3A4 Inducer, and Cytochrome P450 2B6 Inducer, and Cytochrome P450 2C8 Inducer, and Cytochrome P450 2C9 Inducer, and Cytochrome P450 2C19 Inducer, and Organic Anion Transporting Polypeptide 1B1 Inhibitor, and Organic Anion Transporting Polypeptide 1B3 Inhibitor, and Organic Anion Transporter 1 Inhibitor, and Organic Anion Transporter 3 Inhibitor, and Breast Cancer Resistance Protein Inhibitor3,4. It is not indicated for the treatment of patients with wild-type BRAF melanoma or wild-type BRAF NSCLC. MEKINIST is not indicated for the treatment of patients with melanoma who have progressed on prior BRAF-inhibitor therapy5.
| [Synthetic Methods]
The key step in the synthesis of Dabrafenib is the construction of the 1,3-thiazole ring, which is usually carried out by the closing ring directly of thioamide (as a 1,3-binuclear reagent) and anα-carbonyl halide (as a 1,2-amphiphilic reagent). Sulfonyl chloride 1 and aniline 2 gave sulfonamide 3 under basic conditions. Methyl pyrimidine 4 with non-nucleophilic strong alkali LiHMDS pull out the acid proton on the methyl and react with 3 to obtain 5, and the latter has α-bromination with NBS to obtain 1,2-amphiphilic reagent 6, and then 6 reacts with 1 , 3-parent nucleotides 7 to close the ring to obtain 8, and finally reacts with ammonia to obtain Dabrafenib.
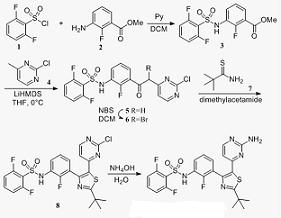
Figure 1: synthetic route of Dabrafenib | [Biological activity]
Dabrafenib (GSK2118436) is a mutant BRAFV600 specific inhibitor with an IC50 of 0.8 nM, and effects for B-Raf (wt) and c-Raf is 4 and 6 fold lower respectively. | [How to use]
It is usually taken twice a day on an empty stomach, 1 hour before or 2 hours after a meal. Take dabrafenib about 12 hours apart at around the same times every day. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Do not stop taking dabrafenib without talking to your doctor.
Swallow the capsules whole; do not split, chew, or crush them.
Your doctor may adjust your dose of dabrafenib depending on your response to treatment and any side effects that you experience. Talk to your doctor about how you are feeling during your treatment. | [Major Side Effects]
The following side effects are common (occurring in greater than 30%) for patients taking dabrafenib :
- Hyperglycemia
- Hyperkeratosis
- Hypophosphatemia
- Headache
These side effects are less common side effects (occurring in about 10-29%) of patients receiving dabrafenib:
- Fever
- Joint pain
- Papilloma (warts/growths)
- Hair loss
- Hand-foot syndrome (Palmar-planter erythrodyesthesia)
- Increased Alkaline phosphatase
- Rash
- Back pain
- Cough
- Muscle aches
- Constipation
- Nasopharyngitis
| [In vitro]
Dabrafenib is selective for Raf kinases and is 400 times more active against B-Raf than other tested 91% kinases. Dabrafenib inhibits B-RafV600E kinase, resulting in reduced phosphorylation of ERK and inhibition of cell proliferation. The cells stagnate in the G1 phase in cancer cells that specifically encode mutated B-RafV600E. | [In vivo]
Dabrafenib (oral) inhibits the growth of B-RafV600E mutated melanoma (A375P). Dabrafenib (oral) also inhibits tumor growth, subcutaneously injecting colon cancer (Colo205) in immunocompromised mice. | [References]
- https://www.caymanchem.com/product/16989
- https://en.wikipedia.org/wiki/Dabrafenib
- https://pubchem.ncbi.nlm.nih.gov/compound/Dabrafenib
- Menzies, A. M., and G. V. Long. "Dabrafenib and trametinib, alone and in combination for BRAF-mutant metastatic melanoma. " Clinical Cancer Research 20.8(2014): 2035-2043.
- https://www.hcp.novartis.com/products/tafinlar-mekinist/
|
|
|