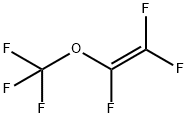
| Identification | Back Directory | [Name]
Trifluoromethyl trifluorovinyl ether | [CAS]
1187-93-5 | [Synonyms]
Hexafluoro-2-oxa-3-butene
Hexafluoro-3-oxa-1-butene
Perfluoro(methylvinylether)
perfluorinatedmethylvinylether
(Trifluoromethoxy)trifluoroethene
Perfluoromethylvinyl Ether (PMVE)
trifluoro(trifluoromethoxy)-ethen
trifluoro(trifluoromethoxy)-Ethene
perfluorovinylperfluoromethylether
PERFLUOROMETHYLPERFLUOROVINYLETHER
ethene,trifluoro(trifluoromethoxy)-
ether,trifluoromethyltrifluorovinyl
trifluoro(trifluoromethoxy)ethylene
TRIFLUOROMETHYL TRIFLUOROVINYL ETHER
Trifluoromethyltrifluorovinylether99%
Trifluoromethyl trifluorovinyl ether 99%
1-(Trifluoromethoxy)-1,2,2-trifluoroethene
1,1,2-Trifluoro-2-(trifluoromethoxy)ethene | [EINECS(EC#)]
214-703-7 | [Molecular Formula]
C3F6O | [MDL Number]
MFCD00221780 | [MOL File]
1187-93-5.mol | [Molecular Weight]
166.02 |
| Safety Data | Back Directory | [Hazard Codes ]
F | [Risk Statements ]
20 | [Safety Statements ]
23-38 | [RIDADR ]
3153 | [RTECS ]
KO4350000 | [Hazard Note ]
Flammable gas | [TSCA ]
T | [HazardClass ]
GAS, FLAMMABLE |
| Hazard Information | Back Directory | [General Description]
A colorless gas. Heavier than air. Easily liquefied. Contact with the liquid may cause frost bite by evaporative cooling. May asphyxiate by displacing air. A flame may flash back from the source of ignition to the source of a leak. Under prolonged exposure to fire or heat containers may rupture violently and rocket. | [Air & Water Reactions]
Highly flammable. | [Reactivity Profile]
Trifluoromethyl trifluorovinyl ether oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick 1979. p.151-154, 164]. | [Health Hazard]
Vapors may cause dizziness or asphyxiation without warning. Some may be irritating if inhaled at high concentrations. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire may produce irritating and/or toxic gases. | [Fire Hazard]
EXTREMELY FLAMMABLE. Will be easily ignited by heat, sparks or flames. Will form explosive mixtures with air. Vapors from liquefied gas are initially heavier than air and spread along ground. CAUTION: Hydrogen (UN1049), Deuterium (UN1957), Hydrogen, refrigerated liquid (UN1966) and Methane (UN1971) are lighter than air and will rise. Hydrogen and Deuterium fires are difficult to detect since they burn with an invisible flame. Use an alternate method of detection (thermal camera, broom handle, etc.) Vapors may travel to source of ignition and flash back. Cylinders exposed to fire may vent and release flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket. | [Flammability and Explosibility]
Flammable |
|
|