| Identification | More | [Name]
Diethyl ether | [CAS]
60-29-7 | [Synonyms]
ALCOHOL-ETHER
ALCOHOL-ETHER MIXTURE
ETHER METHANOL SOLVENT
(C2H5)2O
1,1’-oxobis(ethane)
1,1’-oxybis-ethan
1,1’-oxybisethane
1,1’-oxybis-Ethane
1,1'-Oxybisethane
1-Ethoxyethane
3-Oxapentane
Aether
Anaesthetic ether
anaestheticether
Anesthesia ether
anesthesiaether
Anesthetic ether
anestheticether
Diaethylaether
Dwuetylowy eter | [EINECS(EC#)]
200-467-2 | [Molecular Formula]
C4H10O | [MDL Number]
MFCD00144265 | [Molecular Weight]
74.12 | [MOL File]
60-29-7.mol |
| Chemical Properties | Back Directory | [Appearance]
Ethyl ether is a colorless, mobile, highly flammable, volatile liquid. Characteristic pungent odor. The
Odor Threshold is 0.63 ppm. | [Melting point ]
-116 °C | [Boiling point ]
34.6 °C(lit.) | [density ]
0.714 | [vapor density ]
2.6 (vs air)
| [vapor pressure ]
28.69 psi ( 55 °C)
| [refractive index ]
n20/D 1.3530(lit.)
| [Fp ]
-40 °F
| [storage temp. ]
Store at RT. | [solubility ]
Soluble in water, miscible with ethanol (96 per cent), with methylene chloride and with fatty oils. It is highly flammable. | [form ]
Liquid | [color ]
max. 10 | [Specific Gravity]
0.714 (20/4℃) ; 0.712 (25℃) | [Odor]
Pungent odor detectable at 0.33 ppm | [Relative polarity]
2.9 | [Stability:]
Stable, but light-sensitive, sensitive to air. May contain BHT (2,6-di-tert-butyl-4-methylphenol) as a stabilizer. Substances to be avoided include zinc, halogens, halogen-halogen compounds, nonmetals, nonmetallic oxyhalides, strong oxidizing agents, chromyl chloride, turpentine oils, turps substitutes, nitrates, metallic chlorides. Extre | [explosive limit]
1.7-36%(V) | [Water Solubility ]
69 g/L (20 ºC) | [FreezingPoint ]
-116.3℃ | [Merck ]
14,3806 | [Henry's Law Constant]
12.50(x 10-4 atm?m3/mol at 25 °C) (Signer et al., 1969) | [Dielectric constant]
4.0(40℃) | [Exposure limits]
TLV-TWA 1200 mg/m3 (400 ppm) (ACGIH
and OSHA); STEL 1500 mg/m3 (500 ppm)
(ACGIH). | [LogP]
0.890 | [Uses]
ethyl ether is a solvent that may cause skin irritation. Although considered a non-comedogenic raw material, it is rarely used in cosmetics. | [CAS DataBase Reference]
60-29-7(CAS DataBase Reference) | [NIST Chemistry Reference]
Ethoxy ethane(60-29-7) | [EPA Substance Registry System]
60-29-7(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
F+,Xn,T | [Risk Statements ]
R12:Extremely Flammable.
R19:May form explosive peroxides.
R22:Harmful if swallowed.
R66:Repeated exposure may cause skin dryness or cracking.
R67:Vapors may cause drowsiness and dizziness.
R39/23/24/25:Toxic: danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed .
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S9:Keep container in a well-ventilated place .
S16:Keep away from sources of ignition-No smoking .
S29:Do not empty into drains .
S33:Take precautionary measures against static discharges .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36/37:Wear suitable protective clothing and gloves . | [RIDADR ]
UN 1155 3/PG 1
| [WGK Germany ]
1
| [RTECS ]
KI5775000
| [F ]
10 | [Autoignition Temperature]
160 °C | [TSCA ]
Yes | [HazardClass ]
3 | [PackingGroup ]
I | [HS Code ]
29091100 | [Safety Profile]
Moderately toxic to
humans by ingestion. Poison experimentally
by subcutaneous route. Moderately toxic by
intraperitoneal and intravenous routes.
badly toxic by inhalation. Human systemic
effects by inhalation: olfactory changes.
Mutation data reported. A severe eye and
moderate skin irritant. Ethyl ether is not
corrosive or dangerously reactive. It must
not be considered safe for indlviduals to
inhale or ingest. It is a depressant of the
central nervous system and is capable of
producing intoxication, drowsiness, stupor,
and unconsciousness. Death due to
respiratory failure may result from severe
and continued exposure.
A very dangerous fire and explosion
hazard when exposed to heat or flame. A
storage hazard. It auto-oxidizes to form explosive polymeric 1 -oxy-peroxides.
Explosive reaction with boron triazide,
bromine trifluoride, bromine pentafluoride,
perchloric acid, uranyl nitrate + light, wood
pulp extracts + heat. Violent reaction or
igmtion on contact with halogens (e.g.,
bromine, chlorine), interhalogens (e.g.,
iodine heptafluoride), oxidants (e.g., silver
perchlorate, nitrosyl perchlorate, nitryl
perchlorate, chromyl chloride, fluorine
nitrate, permanganic acid, nitric acid,
hydrogen peroxide, peroxodisulfuric acid,
iodine(VⅡ) oxide, solum peroxide, ozone,
and liquid air), sulfur and sulfur compounds
(e.g., sulfur when dried with peroxidzed
ether, sulfuryl chloride). Can react
vigorously with acetyl peroxide, air,
bromoazide, ClF3, CrO3, Cr(OCl)2, LiAlH2,
NOClO4,02, NClO2, (H2so4 +
permanganates), K2O2, [(C2H5)3di + air],
[(CH3)d + air]. To fight fire, use alcohol
foam, CO2, dry chemical. Used in
production of drugs of abuse. When heated
to decomposition it emits acrid smoke and
irritating fumes. See also ETHERS. | [Hazardous Substances Data]
60-29-7(Hazardous Substances Data) | [Toxicity]
LD50 oral (rat) 1215 mg/kg
LC50 inhal (rat) 73,000 ppm (2 h)
PEL (OSHA) 400 ppm
STEL (ACGIH) 500 ppm | [IDLA]
1,900 ppm [10% LEL] |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Ethanol-->Sulfuric acid-->Sodium metabisulfite-->Diethyl sulfate-->Dehydrolyzing agent-->Ethylene | [Preparation Products]
Allyl methyl carbonate-->2-Methoxyphenylboronic acid-->2-Methoxy-5-pyridineboronic acid-->2-Bromo-4-nitro-1-(trifluoromethoxy)benzene-->(6-ETHOXYPYRIDIN-3-YL)BORONIC ACID-->Tert-butyl bis(2-chloroethyl)carbamate-->2-Amino-6-methyl-4-pyrimidinol-->2,2':5',2''-TERTHIOPHENE-->2,6-Difluorophenylboronic acid-->ethyl 1-methylpyrrole-2-carboxylate-->Benzo[b]thien-2-ylboronic acid-->3-Methylbenzylamine-->4-tert-Butylbenzoyl chloride-->4-(Trifluoromethyl)phenacyl bromide-->1,2-(Methylenedioxy)-4-nitrobenzene-->7-OXABICYCLO[2.2.1]HEPTANE-->N,N,N',N'-TETRAMETHYL-2-BUTENE-1,4-DIAMINE-->1,2-Epoxyoctane-->17beta-Hydroxy-17-methylandrosta-4,9(11)-dien-3-one-->3-(4-BROMOPHENOXY)PROPANOIC ACID-->2-Acetylthiazole-->A-MELANOCYTE STIMULATING HORMONE SYNTHETIC-->2-(4-Ethoxyphenyl)-2-methylpropanol-->N,N-diethyl-1-propynylamine-->Daidzein-->LACTAMIDE-->6-MORPHOLINONICOTINALDEHYDE-->2-PHENYL-2-PROPANOL-->1-Methyl-1H-pyrazole-5-carboxylic acid-->2,4-Dibromothiophene-->2-[5-(Benzyloxy)-1H-indol-3-yl]-2-oxoacetic acid ,97%-->2-Bromo-1-indanone-->ETHYLENEDIAMINE DIACETATE-->1,5-HEXADIENE-->Borane-pyridine complex-->Phosphomolybdic Acid-->Levonorgestrel-->ECGONINE HYDROCHLORIDE AMINO ALCOHOL POR TION-->methylated albumin-->Genistein |
| Hazard Information | Back Directory | [General Description]
A clear colorless liquid with an anesthetic odor. Flash point-49°F. Less dense than water and slightly soluble in water. Hence floats on water. Vapors are heavier than air. Used as a solvent and to make other chemicals. | [Reactivity Profile]
Occasional explosions have occurred when aluminum hydride was stored in ether. The explosions have been blamed on the presence of carbon dioxide impurity in the ether, [J. Amer. Chem. Soc. 70:877(1948)]. Diethyl ether and chromium trioxide react violently at room temperature. Solid acetyl peroxide in contact with ether or any volatile solvent may explode violently. A 5-gram portion in ether detonated while being carried, [Chem. Eng. News 27:175(1949)]. Nitrosyl perchlorate ignites and explodes with diethyl ether. A mixture of ether and ozone forms aldehyde and acetic acid and a heavy liquid, ethyl peroxide, an explosive, [Mellor 1:911(1946-1947)]. | [Air & Water Reactions]
Highly flammable. Oxidizes readily in air to form unstable peroxides that may explode spontaneously [Bretherick, 1979 p.151-154, 164]. A mixture of liquid air and DIETHYL ETHER(60-29-7) exploded spontaneously, [MCA Case History 616(1960)]. | [Hazard]
CNS depressant by inhalation and skin
absorption. Very flammable, severe fire and explosion hazard when exposed to heat or flame. Forms
explosive peroxides. Explosive limits in air 1.85–
48%. | [Health Hazard]
Vapor inhalation may cause headache, nausea, vomiting, and loss of consciousness. Contact with eyes will be irritating. Skin contact from clothing wet with the chemical may cause burns. | [Potential Exposure]
Ethyl ether is used as a solvent for
waxes, fats, oils, perfumes, alkaloids, dyes, gums, resins,
nitrocellulose, hydrocarbons, raw rubber, and smokeless
powder. It is also used as an inhalation anesthetic; a refrigerant; in diesel fuels; in dry cleaning; as an extractant; and
as a chemical reagent for various organic reactions | [Fire Hazard]
Behavior in Fire: Vapor is heavier than air and may travel considerable distance to a source of ignition and flash back. Decomposes violently when heated. | [First aid]
If this chemical gets into the eyes, remove any
contact lenses at once and irrigate immediately for at least
15 minutes, occasionally lifting upper and lower lids. Seek
medical attention immediately. If this chemical contacts the
skin, remove contaminated clothing and wash immediately
with soap and water. Seek medical attention immediately.
If this chemical has been inhaled, remove from exposure,
begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if
heart action has stopped. Transfer promptly to a medical
facility. When this chemical has been swallowed, get medical attention. Give large quantities of salt water and induce
vomiting. Do not make an unconscious person vomit | [Shipping]
UN1155 Diethyl ether or Ethyl ether, Hazard
Class: 3; Labels: 3-Flammable liquid | [Incompatibilities]
May form explosive mixture with air.
Incompatible with strong acids; strong oxidizers halogens,
sulfur, sulfur compounds, causing fire and explosion hazard. Can form peroxides from air, heat, sunlight; may
explode when container is unstoppered or otherwise
opened. Attacks some plastics, rubber and coatings. Being
a nonconductor, chemical may accumulate static electric
charges that may result in ignition of vapor. | [Description]
Diethyl ether is a component of starting fluids and is used as
a solvent in the manufacture of synthetic dyes and plastics.
Because of its characteristics, diethyl ether was widely used in
many countries as an anesthetic agent, but was then replaced by
other substances in the 1960s. | [Waste Disposal]
Concentrated waste containing no peroxides-discharge liquid at a controlled rate near a
pilot flame. Concentrated waste containing peroxidesperforation of a container of the waste from a safe distance
followed by open burning. Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal | [Physical properties]
Colorless, hygroscopic, volatile liquid with a sweet, pungent odor. Odor threshold concentration is
330 ppb (quoted, Keith and Walters, 1992). | [History]
Ether was supposedly discovered by Raymundus Lullus (1232–1315) around 1275,
although there is no extant evidence of this in his writings. The discoverer of ether is often
credited to the German physician and botanist Valerius Cordus (1515–1554), who gave the
first description of the preparation of ether in the mid-16th century. Cordus called the substance
oleum vitrioli dulce, which is translated as sweet oil of vitriol. Cordus used sulfuric acid
(oil of vitriol) to catalyze the conversion of alcohol to ether. At approximately the same time
Paracelsus (1493–1541), a Swiss physician who is also cited as a discoverer of ether, observed
that chickens were safely put to sleep by breathing vapors from sweet oil of vitriol. In 1730,
August Siegmund Frobenius changed the name of sweet vitriol to ether. | [Definition]
ChEBI: An ether in which the oxygen atom is linked to two ethyl groups. | [Definition]
diethyl ether: A colourless flammablevolatile ether, C2H5OC2H5; r.d. 0.71;m.p. –116°C; b.p. 34.5°C. It can bemade by Williamson’s synthesis. Itis an anaesthetic and useful organicsolvent. | [Production Methods]
Ether is produced by the dehydration of ethanol using sulfuric acid: 2CH3CH2OH +2H2SO4 → (CH3CH2)2O + H2SO4 + H2O.the temperature of the reaction is carriedout at about 140°C to control for unwanted products.the volatile ether is distilled from themixture. Ether can also be prepared by Williamson synthesis. In this reaction, ethanol reactswith sodium to form sodium ethanolate (Na+C2H5O?). Sodium ethanolate then reacts withchloroethane to form ether and sodium chloride: Na+C2H5O? +C2H5Cl → C2H5OC2H5 +NaCl. Ether is also produced as a by-product in the production of ethanol. | [Flammability and Explosibility]
Diethyl ether is extremely flammable (NFPA rating = 4) and is one of the most dangerous fire hazards commonly encountered in the laboratory, owing to its volatility and extremely low ignition temperature. Ether vapor may be ignited by hot surfaces such as hot plates and static electricity discharges, and since the vapor is heavier than air, it may travel a considerable distance to an ignition source and flash back. Ether vapor forms explosive mixtures with air at concentrations of 1.9 to 36% (by volume). Carbon dioxide or dry chemical extinguishers should be used for ether fires. Diethyl ether forms unstable peroxides on exposure to air in a reaction that is promoted by light; the presence of these peroxides may lead to explosive residues upon distillation. | [Chemical Reactivity]
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent. | [Industrial uses]
Diethyl ether as a
commercial product is available in several grades and is used as an extraction
solvent, reaction solvent, and as a general anesthetic. Ethyl ether is an excellent
solvent for alkaloids, dyes, fats, gums, oils, resins, and waxes. Blends of ethyl
ether and ethanol are excellent solvents for cellulose nitrate used in the manufacture
of guncotton, in collodion solutions and pyroxylin plastics. Ethyl ether is used in
the recovery of acetic acid from aqueous solutions in the cellulose acetate and
plastic industry. It is used as a starter fuel for diesel engines and as a denaturant in
denatured ethanol formulations. Grignard and Wurtz-Fillig synthesis reactions use
diethyl ether as an anhydrous, inert reaction medium. | [Environmental Fate]
Photolytic. The rate constant for the reaction of ethyl ether and OH radicals in the atmosphere at
300 K is 5.4 x 10-12 cm3/molecule?sec (Hendry and Kenley, 1979).
Chemical/Physical. The atmospheric oxidation of ethyl ether by OH radicals in the presence of
nitric oxide yielded ethyl formate as the major product. Minor products included formaldehyde
and nitrogen dioxide. In the absence of nitric oxide, the products were ethyl formate and
acetaldehyde (Wallington and Japar, 1991).
Ethyl ether will not hydrolyze (Kollig, 1993). | [storage]
ether should be used only in areas free of ignition sources (including hot plates, incandescent light bulbs, and steam baths), and this substance should be stored in tightly sealed metal containers in areas separate from oxidizers. Because of the tendency of diethyl ether to form peroxides on contact with air, containers should be dated upon receipt and at the time they are opened. Diethyl ether is generally supplied with additives that inhibit peroxide formation; distillation removes these inhibitors and renders the liquid more prone to peroxide formation. Material found to contain peroxides should be treated to destroy the peroxides before use or disposed of properly. | [Purification Methods]
Usual impurities are water, EtOH, diethyl peroxide (which is explosive when concentrated), and aldehydes. Peroxides [detected by liberation of iodine from weakly acid (HCl) solutions of KI, or by the blue colour in the ether layer when 1mg of Na2Cr2O7 and 1 drop of dilute H2SO4 in 1mL of water is shaken with 10mL of ether] can be removed in several different ways. The simplest method is to pass dry ether through a column of activated alumina (80g Al2O3/700mL of ether). More commonly, 1L of ether is shaken repeatedly with 5-10mL of a solution comprising 6.0g of ferrous sulfate and 6mL of conc H2SO4 in 110mL of water. Aqueous 10% Na2SO3 or stannous chloride can also be used. The ether is then washed with water, dried for 24hours with CaCl2, filtered and dried further by adding sodium wire until it remains bright. The ether is stored in a dark cool place, until distilled from sodium before use. Peroxides can also be removed by wetting the ether with a little water, then adding excess LiAlH4 or CaH2 and leaving to stand for several hours. (This also dried the ether.) Werner [Analyst 58 335 1933] removed peroxides and aldehydes by adding 8g AgNO3 in 60mL of water to 1L of ether, then 100mL of 4% NaOH and shaking for 6minutes. Fierz-David [Chimia 1 246 1947] shook 1L of ether with 10g of a zinc-copper couple. (This reagent is prepared by suspending zinc dust in 50mL of hot water, adding 5mL of 2M HCl and decanting after 20seconds, washing twice with water, covering with 50mL of water and 5mL of 5% cuprous sulfate with swirling. The liquid is decanted and discarded, and the residue is washed three times with 20mL of ethanol and twice with 20mL of diethyl ether). Aldehydes can be removed from diethyl ether by distillation from hydrazine hydrogen sulfate, phenyl hydrazine or thiosemicarbazide. Peroxides and oxidisable impurities have also been removed by shaking with strongly alkaline-saturated KMnO4 (with which the ether was left to stand in contact for 24hours), followed by washing with water, conc H2SO4, water again, then drying (CaCl2) and distillation from sodium, or sodium containing benzophenone to form the ketyl. Other purification procedures include distillation from sodium triphenylmethide or butyl magnesium bromide, and drying with solid NaOH or P2O5. [Beilstein 1 IV 1314.] Rapid purification: Same as for 1,4-dioxane. | [Toxicity evaluation]
Inhalation is the main route of exposure to diethyl ether.
Occupational exposure to diethyl ether may occur through
inhalation and dermal contact with this compound at workplaces
where diethyl ether is used. Exposure to this chemical
may also occur via inhalation of ambient air and ingestion
of contaminated drinking water. Although rare, intentional
(suicidal) exposure is also reported.
The industrial use of diethyl ether may result in its release to
the environment through various waste streams. In air, diethyl
ether will exist as a vapor and will be degraded in the atmosphere
after reacting with hydroxyl and nitrate radicals. Halflives
of these reactions in air are estimated to be 1.2 and
5.8 days, respectively. In soil and water, diethyl ether is expected
to volatilize and biodegradation is likely to be a slow process.
Bioconcentration of diethyl ether in aquatic organisms is low. | [Toxics Screening Level]
The initial threshold screening level for diethyl ether is 12,000 μg/m3 based on a 8 hour averaging time. |
| Questions And Answer | Back Directory | [Chemical Properties]
Diethyl ether is inactive at room temperatures, but some reactions may also occur. Peroxides are prone to prolonged exposure to oxygen (or light) to become ether peroxide (also known as ether hydroperoxide). Peroxide ether is a viscous liquid that hardly evaporates. Antioxidants are often added in storage of ether to avoid the slow oxidation. Diethyl ether will crack in case of being oxidized violently. In the presence of catalysts, it can break down into aldehydes or acids. It reacts with an organic acid anhydride to form an ester in the presence of a catalyst, or reacts with an inorganic acid anhydride to form an ester without any catalyst. It can react with metal halides to produce addition compounds such as cerium chloride adduct 2(C2H5)2O•BeCl2. It can react with halogen to produce monohalogenated ethers and polyhaloethers. Ether can react with sulfuric acid generating an adduct.
| [Medical uses]
It can be used to test the arm-to-lung blood circulation time. After being injected into the upper arm vein, the drug liquid goes from the right atrium, passes right ventricle to reach the lungs, and is then discharged from the respiratory tract. It normally takes 4 to 6 seconds for the patients to smell ether odor from the infusion moment (or 3 to 8 seconds).
【Usage and Dosage】
- Take 1ml of ether and 2ml of 0.9% sodium chloride solution, mix them and then inject from the arm vein.
- Adverse reactions such as temporary chest discomfort, cough, and local pain may occur.
【Precautions】
- Patients with potential heart failure are banned.
- Do not inject Diethyl ether outside the blood vessels mistakenly.
- Exposure to air or in storage for long, ether forms an explosive mixture of ether peroxides and aldehydes etc.
【Specifications】 Injection: 3ml.
【W(wǎng)arning】
- Patients with severe intracranial hypertonia, acute inflammation of the upper respiratory tract, active tuberculosis, severe respiratory disease, cardiovascular disease, liver and kidney functional impairment, severe metabolic disorders and uncontrolled diabetes are strictly prohibited for Ether anesthesia. If the administration is excessive during the operation, respiratory dangers such as weakness of breathing, fall of blood pressure, rapid pulse and pupil dilation will occur.
- Inhalation at 10% concentration can result in death. The maximum allowable concentration in the workplace is 400×10-6.
| [First-aid]
- Rinse with soap when contact with eyes and skin.
- Help with the breathing using oxygen gas containing 5% carbon dioxide when breathing is abnormal and the face turns blue.
- Drink hot tea and coffee to prevent vomiting.
| [Production]
Diethyl ether is produced by dehydrating ethanol at 300 °C in the presence of catalyst.
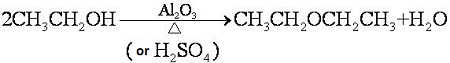 |
|