| Identification | Back Directory | [Name]
Limonin | [CAS]
1180-71-8 | [Synonyms]
EVODIN
LiMone
LIMONIN
LIMONINE
LIMONIN(P)
LIMONIN(RG)
CITROLIMONIN
OBACULACTONE
Evodia lactone
DICTAMNOLACTONE
LIMONIN(P)(CALL)
Limonoate D-ring-lactone
limonin from citrus seeds
Limonoic acid, di-δ-lactone
limonoic acid di-delta-lactone
7,16-Dioxo-7,16-dideoxylimondiol
LIMONOIC ACID 3,19:16,17 DILACTONE
LiMonin, froM TetradiuM ruticarpuM
Limonin,Limonoic acid 3,19:16,17 dilactone
Limonin����,Limonoate D-ring-lactone�����,Limonoic acid di-delta-lactone
LiMonin SynonyMs LiMonoate D-ring-lactone
11H,13H-Oxireno[d]pyrano[4',3':3,3a]isobenzofuro[5,4-f][2]benzopyran-4,6,13(2H,5aH)-trione,8-(3-furyl)decahydro-2,2,4a,8a-tetramethyl-
11H,13H-Oxireno[d]pyrano[4',3':3,3a]isobenzofuro[5,4-f][2]benzopyran-4,6,13(2H,5aH)-trione,8-(3-furanyl)decahydro-2,2,4a,8a-tetramethyl-,(2aR,4aR,4bR,5aS,8S,8aS,10aR,10bR,14aS)- | [Molecular Formula]
C26H30O8 | [MDL Number]
MFCD00075922 | [MOL File]
1180-71-8.mol | [Molecular Weight]
470.51 |
| Chemical Properties | Back Directory | [Melting point ]
298 C | [alpha ]
D -128° (c = 1.21 in acetone) | [Boiling point ]
67 °C | [density ]
1.39±0.1 g/cm3(Predicted) | [storage temp. ]
2-8°C
| [solubility ]
Chloroform (Slightly), DMSO (Sparingly) | [form ]
neat | [color ]
White to Off-White | [λmax]
285nm(lit.) | [Merck ]
13,5516 | [LogP]
0.474 (est) | [EPA Substance Registry System]
11H,13H-Oxireno[ d]pyrano[4',3': 3,3a]isobenzofuro[5,4-f][ 2]benzopyran-4,6,13(2H,5aH)- trione, 8-(3-furanyl)decahydro-2,2, 4a,8a-tetramethyl-, (2aR,4aR,4bR,5aS,8S,8aS,10aR, 10bR,14aS)-(1180-71-8) |
| Hazard Information | Back Directory | [Chemical Properties]
White powder | [Definition]
ChEBI: Limonin is a limonoid, an epoxide, a hexacyclic triterpenoid, a member of furans, an organic heterohexacyclic compound and a lactone. It has a role as a metabolite, an inhibitor and a volatile oil component. | [General Description]
Limonin is a limonoid glycoside with cancer chemo-preventive activity. | [Biochem/physiol Actions]
Limonin is a triterpenoid aglycone that is a bitter principle of citrus fruits. It has anti-proliferative, proapoptotic activity on several cancer cell lines and inhibits azoxymethane-induced colon cancer in rats. It also inhibits HIV-1 replication in culturedf monocytes, macrophages, and mononuclear cells, perhaps by inhibition of HIV-1 protease activity. |
| Questions And Answer | Back Directory | [description]
Limonin is a triterpenoid enriched in citrus fruits, which has antivirus and antitumor ability. Limonin is a phytonutrient found in most citrus fruits. Natural sources of limonin include oranges, grapefruits, lemons, and limes. Within the fruit it is attached to a sugar and the combined molecule is called limonin glucoside.Limonin is what remains after our bodies cleave a glucose, or sugar molecule, from limonin's parent compound, limonin glucoside. Limonin glucoside is present in citrus and citrus juices in about the same amount as vitamin C.
The good news is limonin has shown to have exceptional health benefits in humans and other animals. It seems to be able to help reduce the development and growth of cancers. It also has the effect of lowering cholesterol in the blood stream.
In 2000, Manners, Hasegawa, and their Canadian co-authors reported that limonin may be among the citrus-juice compounds that lower cholesterol. In lab tests, they found that human liver cells produced less apo B—a compound associated with higher cholesterol levels—when exposed to limonin. | [Limonoid compounds]
Limonoid is a kind of triterpenoid derivatives widely distributed in Rutaceae and Meliaceae. They are one of the bitter components of citrus juice, presented in the form of glucose derivatives in mature fruits. Plant seed is the main source of these compounds aglycones and glucoside with grape seeds containing the highest content. It has been measured of 15 kinds of commercial orange juice samples, the average citric acid concentration was (320 ± 48) mg/kg while the value for 8 kinds of grape juices is (190±36) mg/kg and for four kinds of lemon juices is (83 ± 10) Mg/kg. Citrus juice has a very low aglycone content, generally being only about 2mg/kg (Hasegawa 1994).
The molecular structure of these compounds is that the D ring contains furan molecules, which can induce glutathione S-transferase. From the view of the effect of specific compound, the effect of limonin is not as strong as nomilin; the former one have no effect on this liver enzyme inactive while the latter one can induce the enzyme even at a concentration of as low as 5mg. Both of them have higher activity in the small intestine than in the liver. Norilin can inhibit the formation of benzo [a] pyrene-DNA adducts in the liver and target tissues and inhibit benzopyrene-induced lung cancer. In the mice trial, for the initial period, the effect of Norilin in inducing skin cancer through inhibiting DMBA is stronger than that of the limonin while the effect becomes weaker for the former one in the promotion phase. Other structurally related limonoids including Ichangin, isoobacunor C and berberine are also good inducers of glutathione-S-transferase (GST).
The above information was collected by Andy of Chemicalbook. | [Physical and chemical properties]
Limonin is a crystal with bitterness (dichloromethane + isopropanol or acetic acid). It is hard to be dissolved in water and ether, but being soluble in methanol, ethanol and acetic acid. Melting point 298 ° C, optical rotation-128 ° (c = 1.21, acetone).
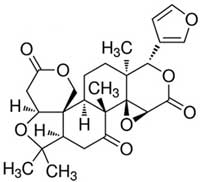
The chemical structure of Limonin | [Pharmacological effects]
Limonoids have various kinds of biological activities such as anti-tumor, insect antifeedant, anti-virus, analgesic, anti-inflammatory and hypnotic. The physiological activity of the limonoid analogues starts from the study of medicinal plants of the Rhodaceae family.
(1) Analgesic and anti-inflammatory effects
Studies have been conducted on the isolation of limonin from Evodiarutaecarpavar. bodinieri. People have also carried out corresponding study on its physiological effects. For example, experiments found that administration of 100 mg/kg limonene to mice can significantly reduce the number of mice of licking feet. At the same time, through the study on the effect of limonin analogue on the vascular permeability of acetic acid channels, bradykinin induced paw swelling reaction and arachidonic acid-induced ear edema, it was found that the product has significant analgesic and anti-Inflammatory effect.
(2) Anti-anxiety sedation
The hypnotic test of anesthetized mice showed that limonoids such as limonin, nomilin, obarone, 7-aureprolone, 70r-limonol and other limonoids could prolong the sleep time of mice induced by the lithium-chlorine aldose and urethane, in which the compound, nomilin has a strong sedative effect.
(3) Pest control insecticidal activity
Regarding the ingestion inhibition effect of limonin analogues, the activity of the azadirachtin isolated from the seeds of A. zadirach ta indica is high and is effective against over 60 species of insects. Citrus limonin analogues (Auronium, Norilin and limonin) are also active against insects and termites. Development and utilization of these natural originated limonin analogs biological insecticides has great significance.
(4) Other effects
In addition to the above biological effects, it also have many other kinds of physiological effects such as antioxidant activity, antibacterial, inhibiting HIV, lowering cholesterol, significant diuretic effect, improving cardiovascular and cerebrovascular circulation and improving sleep, anti-virus, regulating cytochrome and so on, Of health care functions. | [Clinical application]
The study of limonin analogues was initiated for the purpose of removing them from citrus fruit in order to increase the value of the commodity. However, with the further deepened study on the physiological activities of limonoids, more and more attention has been paid to their recovery, refinement and application in citrus. Limonin analogues have physiological activity of inhibiting tumor growth and should be developed as functional food materials. After many years of research, Microherb has successfully isolated high purity 98% limonoids by advanced chromatographic separation technology, which is widely used in medicine, functional food supplements and biological pesticides. | [Uses]
It has antiproliferative and anti-apoptotic activity against several tumor cell lines and can inhibit the rat colon cancer induced by azoxymethane. By inhibiting the activity of HIV-1 protease, it inhibits the replication of HIV-1 virus in monocytes, macrophages and monocytes. |
|
|