| Identification | More | [Name]
Chloranil | [CAS]
118-75-2 | [Synonyms]
2,3,5,6-TETRACHLORO-1,4-BENZOQUINONE
2,3,5,6-TETRACHLORO-2,5-CYCLOHEXADIENE-1,4-DIONE
2,3,5,6-TETRACHLOROBENZO-1,4-QUINONE
2,3,5,6-TETRACHLORO-P-BENZOQUINONE
2,3,5,6-Tetrachloroquinone
4-CHLORANIL
CHLORANIL
P-CHLORANIL
SPERGON
SPERGON I
SPERGON(R)
TETRACHLORO-1,4-BENZOQUINONE
TETRACHLORO BENZOQUINONE
TETRACHLORO-P-BENZOQUINONE
TETRACHLORO-P-QUINONE
TETRACHLOROQUINONE
VULKLOR(R)
1,2,4,5-tetrachlorobenzoquinone
1,4-Benzoquinone, 2,3,5,6-tetrachloro-
1,4-benzoquinone,tetrachloro- | [EINECS(EC#)]
204-274-4 | [Molecular Formula]
C6Cl4O2 | [MDL Number]
MFCD00001594 | [Molecular Weight]
245.88 | [MOL File]
118-75-2.mol |
| Safety Data | Back Directory | [Hazard Codes ]
Xi,N,Xn | [Risk Statements ]
R36/38:Irritating to eyes and skin .
R50/53:Very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R20:Harmful by inhalation. | [Safety Statements ]
S37:Wear suitable gloves .
S60:This material and/or its container must be disposed of as hazardous waste .
S61:Avoid release to the environment. Refer to special instructions safety data sheet .
S22:Do not breathe dust . | [RIDADR ]
UN 3077 9/PG 3
| [WGK Germany ]
2
| [RTECS ]
DK6825000
| [F ]
8 | [Autoignition Temperature]
>400 °C | [TSCA ]
Yes | [HazardClass ]
9 | [PackingGroup ]
III | [HS Code ]
29147090 | [Hazardous Substances Data]
118-75-2(Hazardous Substances Data) | [Toxicity]
LD50 orally in Rabbit: 4000 mg/kg |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Ferric chloride-->Pentachlorophenol-->Sodium pentachlorophenolate | [Preparation Products]
6-Bromoquinoline-->Michler's ketone-->Permanent Violet RL-->Enoxacin-->Reactive Blue 198-->Direct Blue 106-->7H-DIBENZO[C,G]CARBAZOLE-->polyphenylene (IV)-->REMASTRAL BLUE FFRL-->TRANS-1,3-PENTADIENE-->2,5-BIS(1-AZIRIDINYL)-3,6-DICHLORO-2,5-CYCLOHEXADIENE-1,4-DIONE-->2,3,5,6-TETRACHLOROPHENOL-->3,5-DICHLORO-4-METHOXYBENZOIC ACID-->6-DEHYDRONANDROLONE-->P-DIAZOBENZENESULFONIC ACID-->3-BROMOFLUORANTHENE-->Tetrachloro-o-benzoquinone-->Formic acid-->Diindolo[3,2-b:3',2'-m]triphenodioxazinetrisulfonic acid, 8,18-dichloro-5,15-dihydro-, trisodium salt-->2,9-Triphenodioxazinedisulfonic acid, 6,13- dichloro-3,10-bis[(4-chlorophenyl)amino]-, disodium salt 2,9-triphenodioxazinedisulfonic acid, 6,13-dichloro-3,10-bis[(4-chlorophenyl)am 9-triphenodioxazinedisulfonic acid,6,13-dichloro-3,10-bis[(4-chlorophenyl)amino]- disodium salt 2,9-Triphenodioxazinedisulfonic acid,6,13-dichloro-3,10-bis[(4-chlorophenyl)amino]-,disodium salt-->C.I. Sulphur Blue 12 |
| Hazard Information | Back Directory | [General Description]
Yellow powder with a slight odor. | [Reactivity Profile]
CHLORANIL(118-75-2) is sensitive to excessive light and heat. This chemical is incompatible with strong oxidizing agents. CHLORANIL(118-75-2) reacts with alkalis. | [Air & Water Reactions]
Insoluble in water. | [Hazard]
Skin irritant. | [Fire Hazard]
Flash point data for this chemical are not available; however, CHLORANIL is probably combustible. | [Description]
Chloranil: an important intermediate
Chloranil (2,3,5,6-tetrachloro-2,5-cyclohexadiene-1,4-dione), is extensively used as a fungicide, is an intermediate in the synthesis of medicines and pesticides, and is an oxidizing agent used in organic synthesis, particularly for dye intermediates, in China. The annual production of chloranil in recent years, in China, has been about 2000 tonnes. Chloranil is known for its strong oxidizing properties. It can cause oxidative stress and damage to cells by interacting with cysteine residues of some proteins[1-3]. | [Chemical Properties]
YELLOW-GREEN POWDER | [Uses]
Agricultural fungicide, dye intermediate, reagent. manufacture of electrodes for pH measurement. | [Uses]
antipsoriatic | [Uses]
Tetrachloro-1,4-benzoquinone is a potential intermediate in the process of pentachlorophenol-induced carcinogenicity. | [Definition]
ChEBI: A member of the class of 1,4-benzoquiones that is 1,4-benzoquinone in which all four hydrogens are substituted by chlorines. | [Synthesis Reference(s)]
Tetrahedron, 34, p. 1577, 1978 DOI: 10.1016/0040-4020(78)80185-9 | [reaction suitability]
reagent type: oxidant | [Industrial uses]
Three important chloranil manufacturing sites, the Ody Chemical Plant, Qsd Chemical Plant and Yueh Chemical Plant were selected as typical chloranil producers. Three chloranil product samples were collected, one from each plant, and stored at -20 ℃ before analysis. The different uses of chloranil can lead to distinct differences in the quality and purity of the chloranil produced. Sample Ch1 was made by the Ody Chemical Plant for use as an intermediate in pharmaceutical products such as diuretics and antisterone. It was extracted and purified using a recrystallization process to form a product with 99% purity. However, sample Ch3, produced by Yueh Chemical Plant, is not further purified as it is mainly used as a dye and pesticide intermediate, so it has a purity of only 90%. The purity of sample Ch2, from Qsd Chemical Plant, is between that of Ch1 and Ch3, at 96%. | [Synthesis]
The production of chloranil involves two steps: first, phenol is used as the raw material and converted to hydroquinone; and second, the hydroquinone is converted to chloranil in the presence of chlorine or hydrogen peroxide and hydrochloric acid[2].
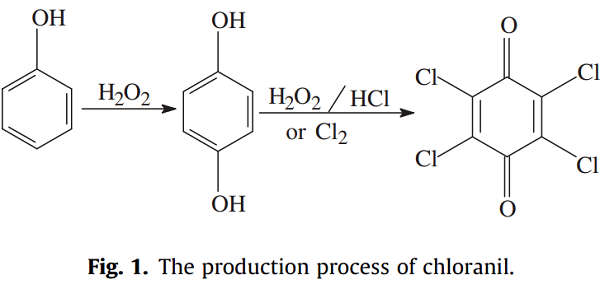 | [Purification Methods]
Crystallise p-chloranil from acetic acid, acetone, *benzene, EtOH or toluene, dry it in a vacuum over P2O5, or from acetic acid and drying over NaOH in a vacuum desiccator. It can be sublimed under vacuum at 290o. A sample may contain significant amounts of the o-chloranil isomer as impurity. Purify it by triple sublimation under vacuum and recrystallise before use. It is a skin and mucous membrane irritant. [UV: Pummerer et al. Chem Ber 85 545 1952, Brook J Chem Soc 5040 1952, Beilstein 7 IV 2083.] | [References]
[1] Wenbin Liu. “Contamination and emission factors of PCDD/Fs, unintentional PCBs, HxCBz, PeCBz and polychlorophenols in chloranil in China.” Chemosphere 86 3 (2012): 248–51.
[2] Kaleru Mogilaiah, Boda Sakram, Janapatla Uma Rani. “Synthesis of 1,2,4-Triazolo[4,3-a][1,8]naphthyridines Using Chloranil under Microwave Irradiation.” ChemInform 37 2 (2005).
[3] Weibing Zhang . “The metabolic activation of pentachlorophenol to chloranil as a potent inhibitor of human and rat placental 3β-hydroxysteroid dehydrogenases.” Toxicology letters 395 (2024): Pages 40-49. |
|
|