| Identification | More | [Name]
TETRAPHENYLSILANE | [CAS]
1048-08-4 | [Synonyms]
TETRAPHENYLSILANE
Silane,tetraphenyl-
tetraphenyl-silan
Tetraphenylsilicon
AIDS019790
tetraphenylsilicane | [EINECS(EC#)]
213-881-3 | [Molecular Formula]
C24H20Si | [MDL Number]
MFCD00014069 | [Molecular Weight]
336.5 | [MOL File]
1048-08-4.mol |
| Chemical Properties | Back Directory | [Appearance]
White solid. Very
stable and inert. Combustible. | [Melting point ]
236 °C | [Boiling point ]
228 °C | [density ]
1.078 | [Fp ]
193°C | [form ]
solid | [color ]
White to Almost white | [Specific Gravity]
1.078 | [Water Solubility ]
Insoluble in water. | [Hydrolytic Sensitivity]
1: no significant reaction with aqueous systems | [BRN ]
1885911 | [Uses]
Heat-transfer medium, polymers. | [CAS DataBase Reference]
1048-08-4(CAS DataBase Reference) | [EPA Substance Registry System]
Silane, tetraphenyl- (1048-08-4) |
| Safety Data | Back Directory | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes . | [TSCA ]
Yes | [HS Code ]
29319090 |
| Hazard Information | Back Directory | [Chemical Properties]
White solid. Very
stable and inert. Combustible. | [Preparation]
Tetraphenylsilane (TPSi) can be obtained by reaction of phenylmagnesium bromide
with tetrachlorosilane or by reaction of phenyllithium with alkoxysilanes.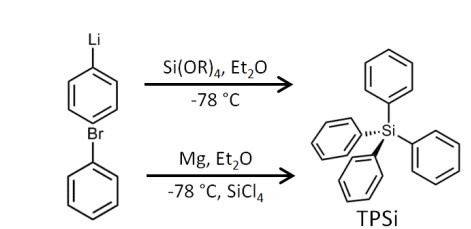
| [Purification Methods]
It crystallises from *benzene as clear colorless bladed needles. It decomposes at ~360o/~760mm on attempted distillation. [George et al. J Am Chem Soc 77 6647 1955, Polis Chem Ber 98 1540 1885, Drew & Landuist J Chem Soc 1480 1935,s Beilstein 16 H 901, 16 I 525, 16 II 606, 16 III 1199, 16 IV 1372.] | [References]
[1] Furgal J, et al. Nucleophilic Attack of R-lithium at Tetrahedral Silicon
in Alkoxysilanes. An Alternate Mechanism. Bulletin of the Chemical Society of Japan, 2016.
|
|
|