| Identification | More | [Name]
1,2-DIPHENOXYETHANE | [CAS]
104-66-5 | [Synonyms]
1,2-DIPHENOXYETHANE
DIPHENYL CELLOSOLVE
DIPHENYL GLYCOL
ETHYLENE GLYCOL DIPHENYL ETHER
(2-Phenoxyethoxy)benzene
1,1’-[1,2-ethanediylbis(oxy)]bis-benzen
1,2-Diphenoxyethan
2-Phenoxyethyl phenyl ether
2-Phenoxyethylphenylether
Benzene,1,1’-[1,2-ethanediylbis(oxy)]bis-
Ethane, 1,2-diphenoxy-
Ethane,1,2-diphenoxy-
Ethylenglykoldiphenylether
ethylene diphenyl ether
1,1'-[1,2-Ethanediylbis(oxy)]bisbenzene
Ethlene diphenyl ether
sym-Diphenoxyethane | [EINECS(EC#)]
203-224-9 | [Molecular Formula]
C14H14O2 | [MDL Number]
MFCD00003039 | [Molecular Weight]
214.26 | [MOL File]
104-66-5.mol |
| Chemical Properties | Back Directory | [Appearance]
Off-white Crystals | [Melting point ]
94-96 °C (lit.) | [Boiling point ]
185 °C / 12mmHg | [density ]
1.0781 (rough estimate) | [vapor pressure ]
0Pa at 25℃ | [refractive index ]
1.5570 (estimate) | [storage temp. ]
Sealed in dry,Room Temperature | [solubility ]
Chloroform (Slightly), Methanol (Slightly) | [form ]
Solid | [color ]
White to Off-White | [Water Solubility ]
22mg/L at 25℃ | [BRN ]
2052248 | [InChIKey]
XCSGHNKDXGYELG-UHFFFAOYSA-N | [LogP]
3.81 at 25℃ | [CAS DataBase Reference]
104-66-5(CAS DataBase Reference) | [EPA Substance Registry System]
2-Phenoxyethyl phenyl ether (104-66-5) |
| Safety Data | Back Directory | [Risk Statements ]
51/53 | [Safety Statements ]
S22:Do not breathe dust .
S24/25:Avoid contact with skin and eyes . | [RIDADR ]
UN 3077 9 / PGIII | [WGK Germany ]
3
| [TSCA ]
Yes | [HS Code ]
29093090 |
| Hazard Information | Back Directory | [Chemical Properties]
Off-white Crystals | [Uses]
1,2-Diphenoxyethane is a reagent that is used in the production and use of activatable delivery compounds containing drugs or a detectable moiety and singlet oxygen-labile linkers. | [Application]
Ethylene glycol diphenyl ether can be used as a new heat-sensitive material sensitizer and a potential polyolefin catalyst additive. | [Preparation]
The preparation of Ethylene glycol diphenyl ether is as follows:A three-necked flask was charged with 15.7 g of bromobenzene (100 mmol) and 47 ml of DMF, and stirred. 12.7 g of sodium carbonate (120 mmol, 1.2 eq.), 1.9 g of cuprous iodide (10 mmol, 0.1 eq.), 1.87 g of 2,2'-bipyridine (12 mmol, 0.1 eq.) and 7.4 g of ethylene glycol (120 mmol, 1.2 equivalent), the reaction mixture was stirred at 100 ° C overnight. After recovering DMF under reduced pressure, 100 ml of water and 100 ml of toluene were added, and the aqueous phase was extracted three times with 300 ml of toluene, and the organic layers were combined. Washed sequentially with 5% sodium carbonate. The organic phase was dried over anhydrous sodium sulfate, filtered, and then filtered and evaporated,The crude product was recrystallized from isopropanol and dried in vacuo to give 19.5 g of product, yield 91%, purity over 99%.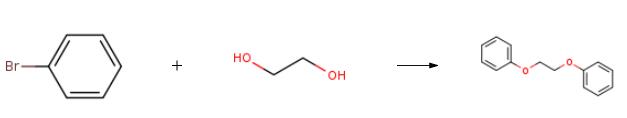
| [General Description]
1,2-Diphenoxyethane is the intermediate formed during sonochemical oxidation of phenoxyacetic acid and chlorophenol. | [Flammability and Explosibility]
Nonflammable | [References]
[1] Jascha Martini . “Structural changes of 1,2-diphenoxyethane upon electronic excitation from a combined Franck-Condon/rotational constants fit.” Journal of Molecular Structure 1270 (2022): Article 133896.
|
| Questions And Answer | Back Directory | [Structure]
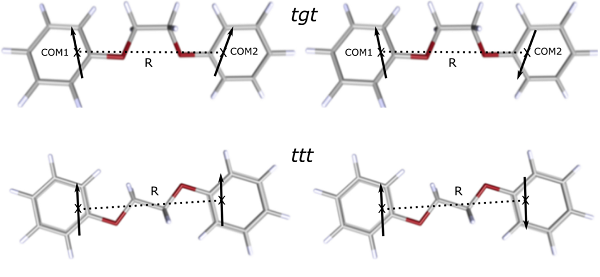
The homo-bichromophoric system 1,2-diphenoxyethane possesses two conformational isomers, which differ in the central O–C–C–O dihedral angle. One, which shows C2 symmetry, can be described as a trans-gauche-trans (tgt) conformer, the other with C2h symmetry as a trans-trans-trans (ttt) conformer. 1,2-diphenoxyethane (C6H5–O–CH2–CH2–O–C6H5) can be viewed as composed of two anisole (C6H5–O–CH2) radical moieties. The excitonic splitting arises from the transition dipole moments (TDM) interaction in the two individual moieties. The excitonic splitting of the two different rotamers of 1,2-diphenoxyethane is different, due to (i) different symmetry, (ii) different distance of the chromophores, and (iii) different orientation of the chromophores. However, the TDM of the individual chromophores is the same for both rotamers. The interaction energy of the two transition dipole moments in 1,2-diphenoxyethane is one wavenumber or less, which is much smaller than the reorganization energy. Vibronic coupling, therefore, is treated as weak coupling[1].
|
|
|