| Identification | Back Directory | [Name]
2-(5-BroMo-2-Methylbenzyl)-5-(4-fluorophenyl)thiophene | [CAS]
1030825-20-7 | [Synonyms]
)-5-(4-fL
Canogliflozin
Canagliflozin-19
Canagliflozin INT4
2-(5-Bromo-2-methyL
Canagliflozin Bromo Imp
Canagliflozin Impurity 52
Canagliflozin Intermediate2
Canagliflozin Bromo Impurity
Canagliflozin Intermediate II
2-(5-bromo-2-methyl-benzyl-(5-fluorophenyl)thiophene
2-(5-BroMo-2-Methylbenzyl)-5-(4-fluorophenyl)thiophene
2-(5-Bromo-2-methylbenzyl)-5-(4-fluorophenyl)thiophene 98%
2-[(5-Bromo-2-methylphenyl)methyl]-5-(4-fluorophenyl)thiophene
2-(4-fluorophenyl)-5-[(5-bromo-2-methylphenyl)methyl]thiophene
Thiophene, 2-[(5-broMo-2-Methylphenyl)Methyl]-5-(4-fluorophenyl)-
2-(5-BroMo-2-Methylbenzyl)-5-(4-fluorophenyl)thiophene 1030825-20-7
1030825-20-7 2-(5-BroMo-2-Methylbenzyl)-5-(4-fluorophenyl)thiophene | [EINECS(EC#)]
807-103-7 | [Molecular Formula]
C18H14BrFS | [MDL Number]
MFCD21496340 | [MOL File]
1030825-20-7.mol | [Molecular Weight]
361.271 |
| Chemical Properties | Back Directory | [Melting point ]
103 °C | [Boiling point ]
438.3±40.0 °C(Predicted) | [density ]
1.388 | [storage temp. ]
Keep in dark place,Sealed in dry,Room Temperature | [solubility ]
DMSO (Slightly, Heated), Hexanes (Slightly, Heated) | [form ]
Solid | [color ]
White to Off-White | [InChI]
InChI=1S/C18H14BrFS/c1-12-2-5-15(19)10-14(12)11-17-8-9-18(21-17)13-3-6-16(20)7-4-13/h2-10H,11H2,1H3 | [InChIKey]
VLRIERSBZHUCOW-UHFFFAOYSA-N | [SMILES]
C1(CC2=CC(Br)=CC=C2C)SC(C2=CC=C(F)C=C2)=CC=1 |
| Hazard Information | Back Directory | [Uses]
2-[(5-Bromo-2-methylphenyl)methyl]-5-(4-fluorophenyl)thiophene is an antidiabetic agent that can be used to prepare Canagliflozin (C175190), a sodium-dependent glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes mellitus. | [Synthesis]
prepares 2-(4-fluorophenyl) thiophene III:
Prepared by Grignard reagent: N 2under protection; magnesium chips (11.7g is added in the 500mL there-necked flask that thermometer, reflux condensing tube, constant pressure funnel be housed; 480mmol), THF (15.0mL), drips 2 p-Fluoro bromo benzenes; add the initiation reaction of 2 iodine post-heating; p-Fluoro bromo benzene (70.0g, 400mmol) is dissolved in THF (250mL), after under reflux, drip the THF solution of p-Fluoro bromo benzene; drip Bi Baowen backflow 2h, GC detection feedstock conversion complete.
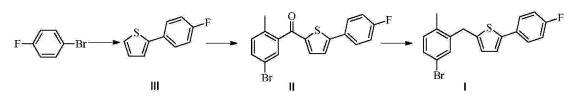
Linked reaction: N 2under protection; in the 1000mL there-necked flask that thermometer, reflux condensing tube, constant pressure funnel be housed, add 2-bromothiophene (52.2g, 320mmol), under stirring, add two (methyl ethyl diketone) palladium (23.4mg; 0.0320mmol); THF (200mL), is heated to 50 DEG C, the above-mentioned Grignard reagent prepared of rear dropping; drip to finish and be warming up to 60 DEG C; insulation 1h, GC detect to raw material reaction complete, terminate reaction.System is cooled to 30 DEG C, drips dilute hydrochloric acid (2M, 150mL) under ice-water bath, drips complete vigorous stirring 0.5h, removes ice-water bath and make system naturally rise to room temperature, stratification, water layer CH 2cl 2(100mL × 3) extract, merge organic phase saturated sodium-chloride 200mL to wash, rear drying, concentrating under reduced pressure obtain light tan solid, vacuum-drying to constant weight obtains 2-(4-fluorophenyl) thiophene III (57.4g, 312mmol), it is 98.5% that GC detects purity, and yield is 99.1%.
1H NMR(500MHz,CDCl 3)δ7.65~7.54(m,2H),7.33~7.22(m,2H),7.14~7.03(m,3H).Gc-Ms:178.1. |
|
|