
Z-TRANS-4-HYDROXY-L-PROLINOL synthesis
- Product Name:Z-TRANS-4-HYDROXY-L-PROLINOL
- CAS Number:95687-41-5
- Molecular formula:C13H17NO4
- Molecular Weight:251.28

64187-48-0
148 suppliers
$14.00/5G

95687-41-5
126 suppliers
$19.00/250mg
Yield:95687-41-5 99%
Reaction Conditions:
with lithium tetrahydridoborate in tetrahydrofuran at 0 - 20; for 4.5 h;
Steps:
1.6
(2S, 4R)-N-LBeLzyloxvcarbonvo-2-hydroxymethyl-4-hydroxvpvrrolidine (6); To a stirred, cold (0 °C) solution of intermediate (5) (58.4 g, 0.208 mol) in THF (400 mL) was added LiBH4 (3.5 g, 0.16 mol) portion-wise. The reaction mixture was stirred for 30 min at (0-5 °C) then for 4 h at room temperature. At this time, TLC (silica gel plates developed in EtOAc) showed just a trace of starting material. The thick reaction mixture was diluted in succession with H20 (250 mL) and 2 M HC1 (277 mL), and then concentrated in vacuo to remove THF. The resulting aqueous concentrate was extracted with EtOAc (4 x 175 mL). Combined EtOAc extracts were washed with brine (2 x 200 mL), dried over MgS04, and then concentrated in vacuo to give intermediate (5) as an oil (51.9 g, 99%). Additional reactions were carried out to give a total of 227 g of product suitable for further transformation. In later preparations the more convenient commercial solution of LiBH4 in THF was used
References:
WO2005/40170,2005,A2 Location in patent:Page/Page column 33-34

13504-85-3
281 suppliers
$5.00/1g

95687-41-5
126 suppliers
$19.00/250mg
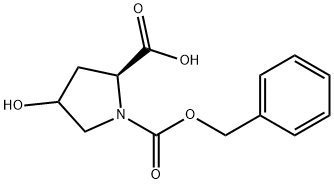
197079-48-4
0 suppliers
inquiry

95687-41-5
126 suppliers
$19.00/250mg

501-53-1
395 suppliers
$10.00/1g

95687-41-5
126 suppliers
$19.00/250mg

51-35-4
845 suppliers
$6.00/5g

95687-41-5
126 suppliers
$19.00/250mg