
L-Hydroxyproline synthesis
- Product Name:L-Hydroxyproline
- CAS Number:51-35-4
- Molecular formula:C5H9NO3
- Molecular Weight:131.13
Today L-Hydroxyproline is manufactured by bio-catalysed hydroxylation of proline in bacteria. Together with its other isomers, L-Hydroxyproline is also used as an inter mediate for a range of pharmaceutical active ingredients.
Produced by hydroxylation of L-proline after protein synthesis. It is contained rich in collagen and elastin. Known to stabilization of triple-helix structure of collagen. Widely used as an intermediate for medicines.
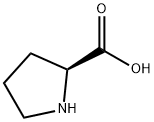
147-85-3
995 suppliers
$6.00/25g

51-35-4
847 suppliers
$6.00/5g
Yield:51-35-4 > 95 %Chromat.
Reaction Conditions:
with hydrogenchloride;α-ketoglutaric acid;ammonium iron (II) sulfate;L-proline trans-4-hydroxylase;sodium L-ascorbate in aq. buffer at 21; pH=6.5; for 14 h;Enzymatic reaction;
Steps:
1.4.5.1. LC/MS Assays
General procedure: Analytical scale proline hydroxylase (PH) incubations were performed by sequential addition of the reagents in Table S1 to a 1.5 mL Eppendorf tube (100 μL final volume): The incubation mixture was kept at 21 °C for 14 h (unless otherwise stated). To quench the reaction, an equal volume of methanol was added and the mixture cooled on ice for 10 min before centrifugation (13,000g for 3 min); the quenching methanol contained 0.25 mM p-aminosalicylic acid (pASA) as an internal standard. The supernatant was decanted and analysed by an LC/MS. ‘Negative controls’ were performed in parallel, but with substitution of 50 mM MES-NaOH, pH 6.5 for the enzyme solution.
References:
Smart, Tristan J.;Hamed, Refaat B.;Claridge, Timothy D.W.;Schofield, Christopher J. [Bioorganic Chemistry,2020,vol. 94,art. no. 103386] Location in patent:supporting information
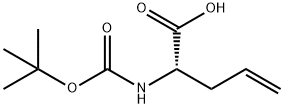
90600-20-7
137 suppliers
$15.00/100mg

51-35-4
847 suppliers
$6.00/5g
![D-threo-Pentonic acid, 5-bromo-2,3,5-trideoxy-2-[[(1,1-dimethylethoxy)carbonyl]amino]-, -lactone](/CAS/20211123/GIF/104241-30-7.gif)
104241-30-7
2 suppliers
inquiry

51-35-4
847 suppliers
$6.00/5g
104241-32-9
0 suppliers
inquiry

51-35-4
847 suppliers
$6.00/5g
104241-31-8
1 suppliers
inquiry

51-35-4
847 suppliers
$6.00/5g