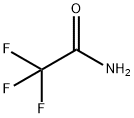
Trifluoroacetamide synthesis
- Product Name:Trifluoroacetamide
- CAS Number:354-38-1
- Molecular formula:C2H2F3NO
- Molecular Weight:113.04
10.1142/S1088424621500292

383-63-1
484 suppliers
$10.00/25g

354-38-1
403 suppliers
$6.00/10g
Yield:354-38-1 100%
Reaction Conditions:
with hydrogenchloride;triethylamine in ethanol;ethyl acetate;
Steps:
5 Synthesis of Atropisomeric Trifluoroacetamide 3a
Example 5 Synthesis of Atropisomeric Trifluoroacetamide 3a Atropisomer 2a as the sulfate salt (0.93 gm, 1.84 mmoles, 503.4 MW) was dissolved in 15 ml ethanol. Triethylamine (1.8 ml, 13 mmoles) and ethyl trifluoroacetate (2.2 ml, 18 mmoles) were slowly added (FIG. 2a). The mixture was stirred at room temperature under argon for 2.5 hours. Volatiles were removed under vacuum and the resulting residue was dissolved in 300 ml ethyl acetate and washed with 2*50 ml of 5% HCl. The ethyl acetate fraction was dried over anhydrous Na2SO4, filtered and concentrated under vacuum to yield atropisomeric trifluoroacetamide 3a as an orange solid (0.92 gm, 100% yield, 501.4 MW). 1H NMR (methanol-d4) δ8.35, 1H, d; 8.15, 1H, d; 7.80, 1H, s; 6.82, 1H, s; 6.65, 4H, m; 4.82, 2H, s.
References:
US6448407,2002,B1
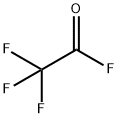
354-34-7
46 suppliers
inquiry

354-38-1
403 suppliers
$6.00/10g

79-37-8
472 suppliers
$17.67/10gm:
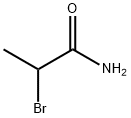
5875-25-2
70 suppliers
$20.00/1g

354-38-1
403 suppliers
$6.00/10g
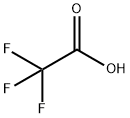
76-05-1
689 suppliers
$9.00/1g

354-38-1
403 suppliers
$6.00/10g
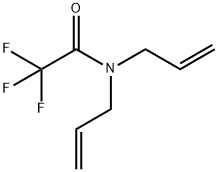
14618-49-6
11 suppliers
$52.20/5ml

354-38-1
403 suppliers
$6.00/10g
