
Trichloroethylene synthesis
- Product Name:Trichloroethylene
- CAS Number:79-01-6
- Molecular formula:C2HCl3
- Molecular Weight:131.39

75-88-7
1 suppliers
inquiry
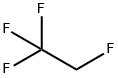
811-97-2
2 suppliers
$45.00/25g

1649-08-7
3 suppliers
inquiry

306-83-2
1 suppliers
$55.00/25g
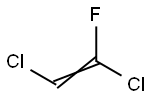
430-58-0
27 suppliers
inquiry

79-01-6
454 suppliers
$19.21/100ml
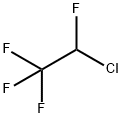
2837-89-0
2 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 1,1,1-trifluoro-2-chloroethanewith hydrogen fluoride;oxygen at 380; under 760.051 Torr;Inert atmosphere;
Stage #2: with hydrogenchloride at 380; under 760.051 Torr;
Steps:
4.2. Catalytic measurements and analysis
The transformation of CF3CH2Cl was carried out under atmospheric pressure in a fixed bed reactor at 380 °C. All the reactants were diluted in helium. The flow rate of CF3CH2Cl was regulated at 50 8C (to avoid any condensation) by a Brooks mass flowmeter. The helium, O2 and HCl feeds were regulated at room temperature by a Brooks mass flowmeter. The catalyst (sieved to a diameter between 0.250 mm and 0.315 mm) was mixed with 6 cm3 of Lonza graphite. When the reaction products emerged from the reactor they were washed successively in two flasks containing water and then in one containing potassium hydroxide (1 M) to neutralize the unreacted hydrogen fluoride and HCl produced by Cl/F exchanges. They were then dried on a 4 ? molecular sieve.
References:
Loustaunau;Fayolle-Romelaer;Celerier;Brunet [Journal of Fluorine Chemistry,2011,vol. 132,# 12,p. 1262 - 1265] Location in patent:experimental part

107-06-2
426 suppliers
$14.00/25g

127-18-4
739 suppliers
$15.00/5G

79-00-5
205 suppliers
$15.00/10ml

79-01-6
454 suppliers
$19.21/100ml
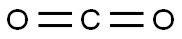
124-38-9
130 suppliers
$214.00/14L
201230-82-2
1 suppliers
inquiry
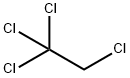
630-20-6
92 suppliers
$58.00/10g

79-01-6
454 suppliers
$19.21/100ml
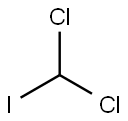
594-04-7
25 suppliers
$1455.00/500mg

79-01-6
454 suppliers
$19.21/100ml
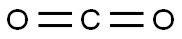
124-38-9
130 suppliers
$214.00/14L
201230-82-2
1 suppliers
inquiry
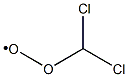
73761-31-6
0 suppliers
inquiry
