Tamoxifen synthesis
- Product Name:Tamoxifen
- CAS Number:10540-29-1
- Molecular formula:C26H29NO
- Molecular Weight:371.51

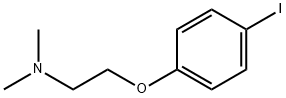
![alpha-[4-[2-(dimethylamino)ethoxy]phenyl]-beta-ethyl-alpha-phenylphenethyl alcohol](/CAS/GIF/748-97-0.gif)
748-97-0
29 suppliers
inquiry

10540-29-1
291 suppliers
$28.00/5mg
Yield:10540-29-1 84.8%
Reaction Conditions:
with hydrogenchloride in lithium hydroxide monohydrate at 60; for 10 h;Industrial scale;Temperature;
Steps:
1.3; 2.3; 3.3 (3) Dehydration and alkalization:
143kg of hydrochloric acid (37wt%) was added to the dehydration kettle, and stirring was started,14.28kg of Grignard was added, the temperature was raised to 60°C, and the reaction was kept for 10h.Then cool down to below 40°C, put the feed liquid into the centrifuge, rinse with purified water twice,Centrifuge again, take out the filter cake; add purified water to the alkalization kettle, turn on stirring,Then add the filter cake, heat up to 50 ° C, stir and dissolve for 30 min, add sodium hydroxide solution (containing 9.43 kg of sodium hydroxide), make the pH value of the reaction system greater than 14, heat up to 55 ° C,Incubate the reaction for 4 hours; after the reaction, discharge the material to a centrifuge for filtration, rinse the filter cake with purified water until the pH of the filtrate is neutral, and then filter for 10 minutes, take out the filter cake, and vacuum dry at -0.090MPa and 60 °C 10h, obtain tamoxifen free base crude product;Add the crude tamoxifen free base and petroleum ether into the refining kettle, stir and dissolve at 45°C for 20min, then add activated carbon, heat up to 80°C, reflux for 30min, and then transfer the feed liquid to the stainless steel crystallization tank, below 0°C Crystallize for more than 10 hours, and then centrifuge. The filter cake after centrifugation is vacuum-dried at -0.090MPa and 70°C to obtain tamoxifen free base, which is white or off-white powder crystal, isomer <0.05%, and other impurities and <0.5%,Weighing 11.53kg, the yield is 84.8%.
References:
CN114133334,2022,A Location in patent:Paragraph 0059; 0064-0065; 0068; 0073-0074; 0077; 0082-0083
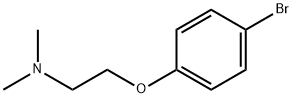
2474-07-9
53 suppliers
$35.20/250mg

20218-41-1
1 suppliers
inquiry

10540-29-1
291 suppliers
$28.00/5mg
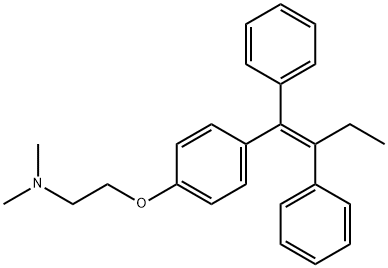
![2-[4-[(E)-1,2-diphenylbut-1-enyl]phenoxy]-N,N-dimethyl-ethanamine](/CAS/GIF/13002-65-8.gif)
13002-65-8
54 suppliers
$289.00/10mg

10540-29-1
291 suppliers
$28.00/5mg
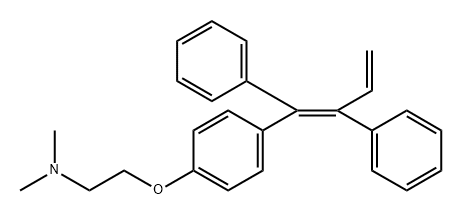
604010-60-8
10 suppliers
inquiry

10540-29-1
291 suppliers
$28.00/5mg
![1,3,2-Dioxaborolane, 2-[(1E)-1,2-diphenyl-1-buten-1-yl]-4,4,5,5-tetramethyl-](/CAS/20210305/GIF/865999-25-3.gif)