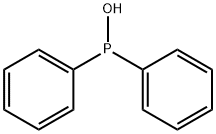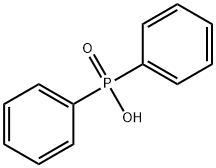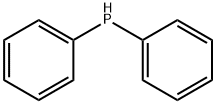
Diphenylphosphine synthesis
- Product Name:Diphenylphosphine
- CAS Number:829-85-6
- Molecular formula:C12H11P
- Molecular Weight:186.19
PPh3 + 2 Li → LiPPh2 + LiPh
LiPPh2 + H2O → Ph2PH + LiOH
Yield:829-85-6 90%
Reaction Conditions:
in neat liquid at 175 - 180;
Steps:
Diphenylphosphine
A two-neck flask equipped with a capillary, a Wurtz adapter,and a descending condenser, was charged with 121.2 g(0.60mol) of diphenylphosphinous acid[29] and 6.5 g (0.03mol)of diphenylphosphinic acid.[18,30] The resulting mixture washeated under vacuum at 175-180 (bath) and on distillationyielded diphenylphosphine (b.p. 156-160 /10 torr). Yield:50.2 g (90%), dP (DMSO): 39.0 ppm, JPH 215.4Hz.The stillage residue was heated at 80-90 with a solutionof 20 g of KOH in 150mL of water until complete dissolution.The resulting mixture was cooled to room temperature, filtered,and acidified with dilute H2SO4 to h 3-4. Theresulting precipitate was filtered off, rinsed with water, anddried in air to give 66.0 g (100%) of diphenylphosphinic acid.M. p. 193-195 (Literature m.p.: 193-195 [18])
References:
Bondarenko, Natalia A.;Tcarkova, Kseniia V.;Belus', Svetlana K.;Artyushin, Oleg I. [Phosphorus, Sulfur and Silicon and the Related Elements,2021,vol. 196,# 10,p. 902 - 910]
1101-41-3
21 suppliers
$90.20/1g

829-85-6
272 suppliers
$33.00/25g
1079-66-9
428 suppliers
$5.00/5g

829-85-6
272 suppliers
$33.00/25g
4559-70-0
321 suppliers
$8.00/5g

829-85-6
272 suppliers
$33.00/25g
7439-96-5
287 suppliers
$22.00/5g

1079-66-9
428 suppliers
$5.00/5g

829-85-6
272 suppliers
$33.00/25g