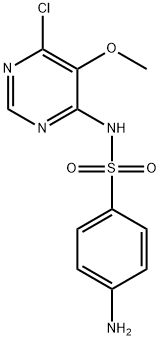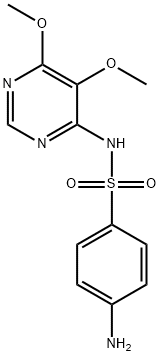
Sulfadoxine synthesis
- Product Name:Sulfadoxine
- CAS Number:2447-57-6
- Molecular formula:C12H14N4O4S
- Molecular Weight:310.33

![sodium [(4-aminophenyl)sulfonyl]azanide](/CAS/20211123/GIF/10103-15-8.gif)
10103-15-8
4 suppliers
inquiry
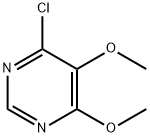
5193-88-4
38 suppliers
$29.00/100mg

2447-57-6
383 suppliers
$5.00/10mg
Yield:2447-57-6 94.4%
Reaction Conditions:
with sodium carbonate in N,N-dimethyl-formamide at 85 - 90; for 4 h;Reagent/catalyst;Temperature;Solvent;
Steps:
2-6 Example 6 Preparation of Sulfadoxine Formula I
Respectively mix DMF 175.0g, sulfa sodium formula III 106.8g (0.55mol), 87.3g (0.50mol) of 4-chloro-5,6-dimethoxypyrimidine formula II and 53.0g of sodium carbonate were added to the reaction flask. After theaddition, the temperature was raised to 85-90 °C and reactedfor 4 hours. After the reaction, the vacuum is turned on, and DMF is recovered by distillation under reduced pressure. After the evaporation iscompleted, water is added. After stirring to dissolve, adjust the pH=7-8 with dilute acetic acid, cool, filter, and the filter cake can recover the sulfonamide. Transfer the filtrate to another reaction flask, adjust the pH=5.1-5.4 with dilute acetic acid, filtered, washed, and dried to obtain 146.5 g of sulfadoxine formula I, with a yield of 94.4%; HPLC purity 99.8%, without condensate formula VI impurities.
References:
CN112457259,2021,A Location in patent:Paragraph 0033-0047