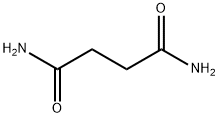
SUCCINAMIDE synthesis
- Product Name:SUCCINAMIDE
- CAS Number:110-14-5
- Molecular formula:C4H8N2O2
- Molecular Weight:116.12

110-61-2
230 suppliers
$13.00/5g

110-14-5
111 suppliers
$10.00/1g
Yield:110-14-5 61%
Reaction Conditions:
Stage #1:butanedinitrile with acetamide;acetic acid at 50; under 760.051 Torr; for 0.5 h;
Stage #2: with tetrakis(acetonitrile)palladium(II) tetrafluoroborate at 50; under 1 - 3 Torr; for 2 h;
Steps:
Typical procedure: Hydration of 1a to 2a
General procedure: To a 500-mL round-bottomed flask equippedwith a stirring bar and a 2-necked Teflon stopcock were added 1a (942.1 mg, 10.0 mmol),acetamide (5900.5 mg, 99.9 mmol), and acetic acid (20 mL). The mixture was stirred at50 C for 30 min under open air (1 atm). Pd(CH3CN)4(BF4)2 (4.52 mg, 0.0102 mmol) wasadded to start the reaction, and the mixture was stirred at 50 °C for 2 h under reducedpressure (1–3 mmHg). The internal pressure was continuously reduced using a belt driverotary vane vacuum pump (SATO VAC INC. USW-50) equipped with a 450-mL liq.nitrogen trap (for acetonitrile and acetic acid, Figure S2). The resulting pale-yellow crudemixture was washed with acetonitrile (50 mL) with sonication for 1 h to removeacetamide. The precipitate was collected by filtration on a membrane filter (MerckMillipore JHWP04700 0.45 μm pore size, hydrophilic PTFE membrane, 47 mmdiameter) and dried in vacuo at 120 C overnight to afford 2a (1043.3 mg, 80percent yield)with minor contamination with acetamide (27.0 mg, as determined by 1H NMR). Theproduct (499.7 mg) was recrystallized from methanol to give analytical pure 2a (418.9mg, 69percent overall yield).
References:
Naka, Hiroshi;Naraoka, Asuka [Synlett,2019,vol. 30,# 17,p. 1977 - 1980] Location in patent:supporting information
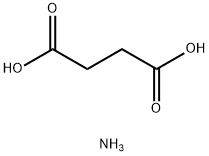
2226-88-2
172 suppliers
$345.00/100g

123-56-8
492 suppliers
$6.00/25g

110-14-5
111 suppliers
$10.00/1g
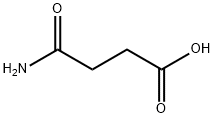
638-32-4
46 suppliers
$21.00/1g

123-56-8
492 suppliers
$6.00/25g

110-14-5
111 suppliers
$10.00/1g
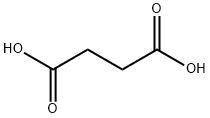
110-15-6
955 suppliers
$5.00/10g

110-14-5
111 suppliers
$10.00/1g