
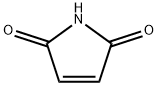
Succinimide synthesis
- Product Name:Succinimide
- CAS Number:123-56-8
- Molecular formula:C4H5NO2
- Molecular Weight:99.09
Recent Japanese patents suggested inert aprotic solvents such as cyclic amides (e.g., N-methylpyrrolidone, N-acetyl-2-pyrrolidone, DMF, or tet-ramethylurea) and azeotroping solvents (e.g., toluene, 0-xylene, hexane, or pentane) or other high-boiling solvents (e.g., chlorobenzene or chlo-rotoluene) as dehydrating agents for the formation of imides from dibasic acids (e.g., 3- and/or 4) hydroxyphthalic acid with a large variety of diamines including diaminosiloxanes such as bis (3-aminopropyl)-tetra-methylsiloxane.

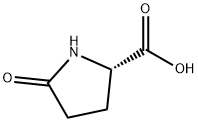
98-79-3
610 suppliers
$5.00/25g

123-56-8
487 suppliers
$6.00/25g
Yield:123-56-8 99%
Reaction Conditions:
with ammonium peroxydisulfate;silver nitrate in water at 20; for 2 h;Reagent/catalyst;Temperature;
Steps:
2
0.13 g of pyroglutamic acid, 1mmol was placed in the reaction tube ,AgNO3 (1.7 mg, 0.01 mmol) was weighed, (NH4)2S2O8 (0.50 g, 2.2 mmol) was added, 3 mL of water was added and stirred at room temperature for 2h. After completion of the reaction, the reaction mixture was concentrated to dryness under reduced pressure to give succinimide. The prepared succinimide was added to deuterated chloroform,Filter, test succinimideThe NMR spectrum is shown in Fig.1
References:
CN103804269,2016,B Location in patent:Paragraph 0036-0041
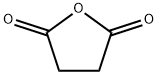
108-30-5
628 suppliers
$5.00/25g

123-56-8
487 suppliers
$6.00/25g
872-50-4
906 suppliers
$10.00/100ML
617-45-8
426 suppliers
$5.00/25g

123-56-8
487 suppliers
$6.00/25g
541-59-3
364 suppliers
$8.00/5g

123-56-8
487 suppliers
$6.00/25g

56-84-8
878 suppliers
$5.00/25g

123-56-8
487 suppliers
$6.00/25g