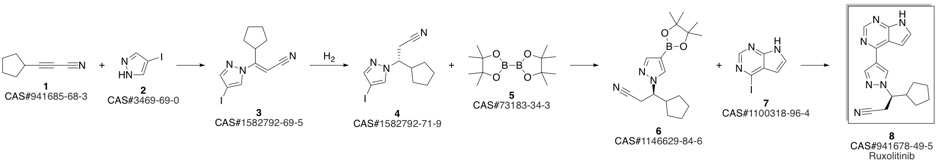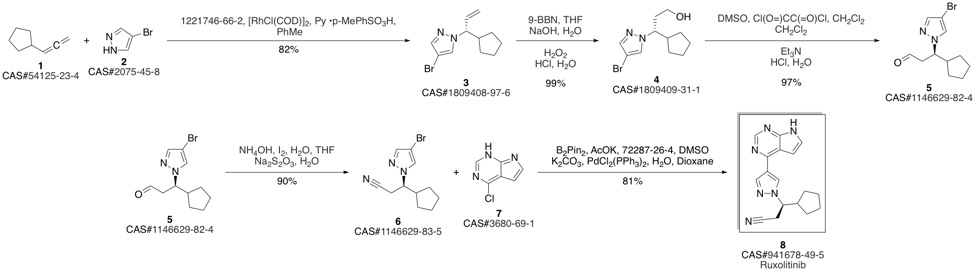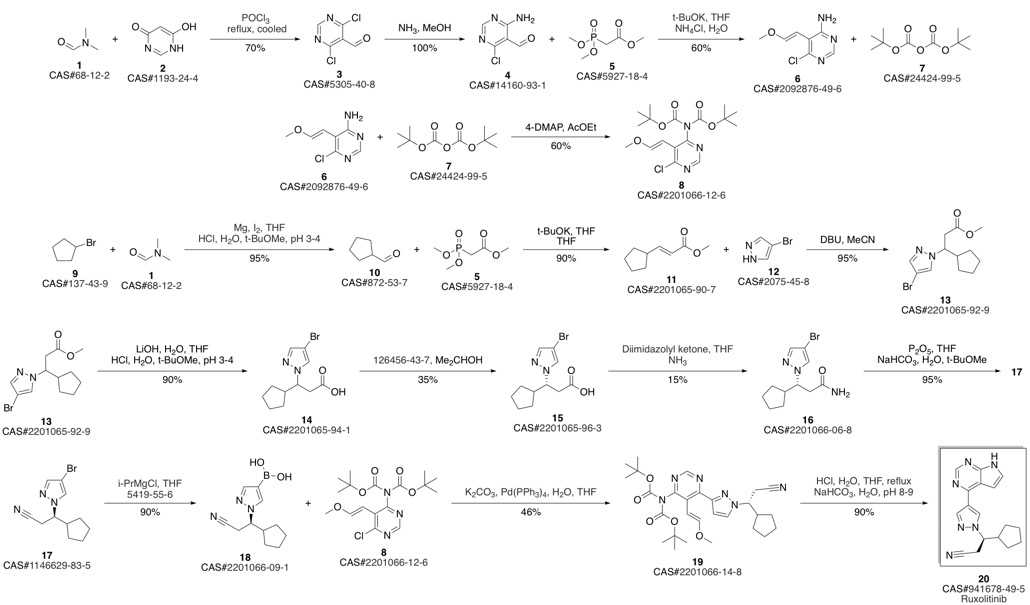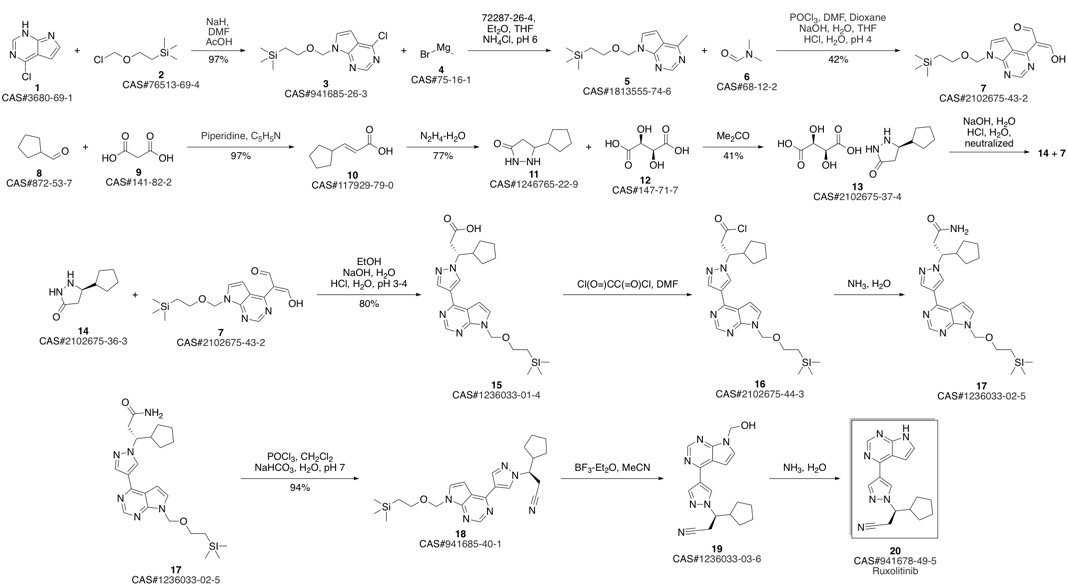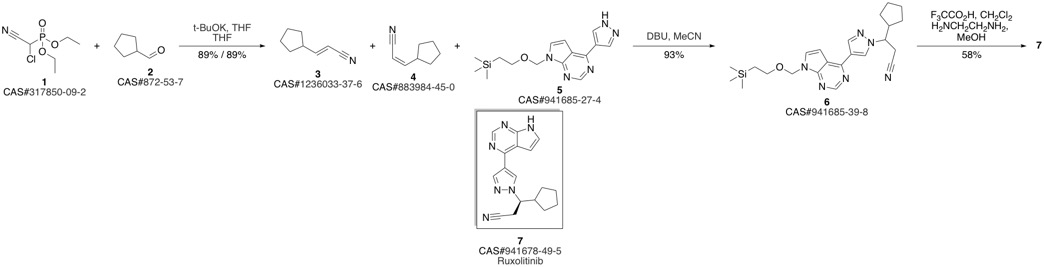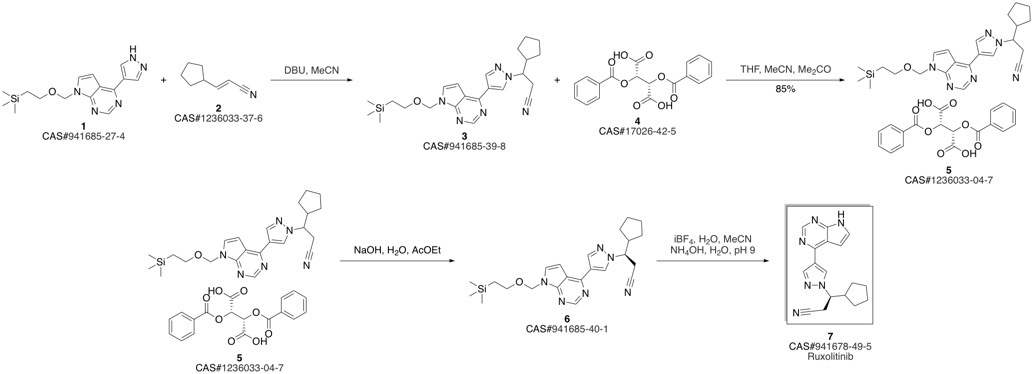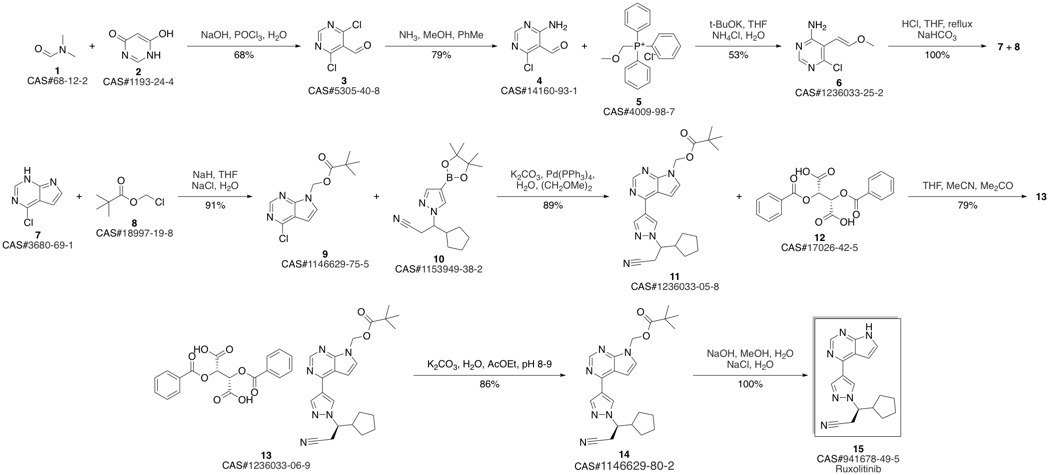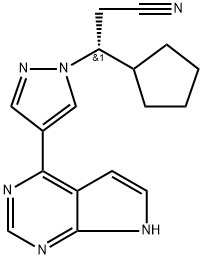
Ruxolitinib synthesis
- Product Name:Ruxolitinib
- CAS Number:941678-49-5
- Molecular formula:C17H18N6
- Molecular Weight:306.37
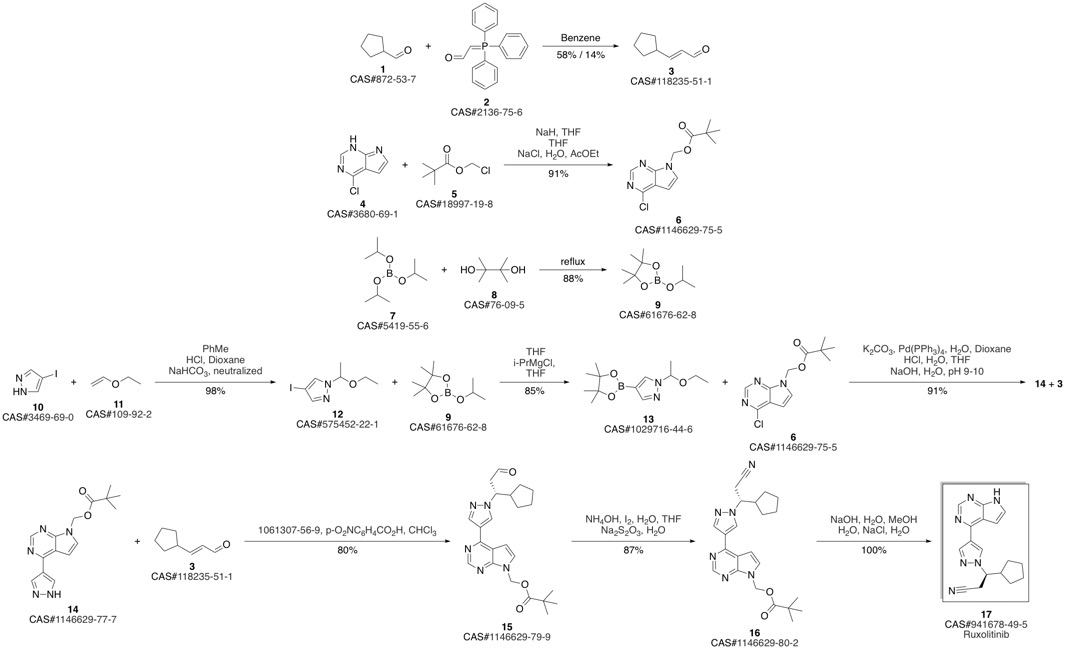
Lin, Qiyan; Meloni, David; Pan, Yongchun; Xia, Michael; Rodgers, James; Shepard, Stacey; Li, Mei; Galya, Laurine; Metcalf, Brian; Yue, Tai-Yuen; Liu, Pingli; Zhou, Jiacheng. Enantioselective synthesis of Janus kinase inhibitor INCB018424 via an organocatalytic aza-?Michael reaction. Jiacheng. Organic Letters. Volume 11. Issue 9. Pages 1999-2002. Journal. (2009).
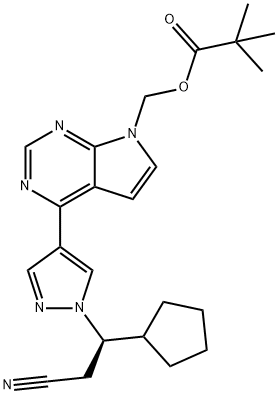
1146629-80-2
10 suppliers
inquiry

941678-49-5
366 suppliers
$28.00/1mg
Yield:941678-49-5 100%
Reaction Conditions:
with water;sodium hydroxide in methanol at 20;Product distribution / selectivity;
Steps:
B
(3R)-Cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-1-yl]propionitrile ((R)-12, free base).; Method B.; To a stirred solution of (4-{1-[(1R)-2-cyano-1-cyclopentylethyl]-1H-pyrazol-4-yl}-7H-pyrrolo [2,3-d]pyrimidin-7-yl)methyl pivalate ((R)-21, 376 mg, 0.894 mmol) in methanol (4.0 mL, 99 mmol) at room temperature was added 1.0 M solution of sodium hydroxide in water (NaOH, 179 μL, 0.179 mmol, 2.0 equiv). The reaction mixture was stirred at room temperature for overnight (15 h). When LCMS showed the reaction was done cleanly, the reaction mixture was quenched with water (10 mL) and saturated aqueous NaCl solution (20 mL), and extracted with EtOAc (2×10 mL). The combined organic layers were washed with brine, dried over magnesium sulfate, filtered and concentrated under reduced pressure to afford (3R)-cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-1-yl]propionitrile ((R)-12, free base, 274 mg, 274 mg theoretical, 100% yield) as a pale yellow foam, which was found to be identical as the material made from Method A.
References:
US2010/190981,2010,A1 Location in patent:Page/Page column 87-88
![1H-Pyrazole-1-propanenitrile, β-cyclopentyl-4-[7-[[2-(triMethylsilyl)ethoxy]Methyl]-7H-pyrrolo[2,3-d]pyriMidin-4-yl]-, (βR)-](/CAS/20180808/GIF/941685-40-1.gif)
941685-40-1
19 suppliers
inquiry

941678-49-5
366 suppliers
$28.00/1mg
![1H-Pyrazole-1-propanenitrile, β-cyclopentyl-4-[7-[[2-(triMethylsilyl)ethoxy]Methyl]-7H-pyrrolo[2,3-d]pyriMidin-4-yl]-, (βR)-](/CAS/20180808/GIF/941685-40-1.gif)
941685-40-1
19 suppliers
inquiry

941678-49-5
366 suppliers
$28.00/1mg
![1H-Pyrazole-1-propanenitrile, β-cyclopentyl-4-[7-(hydroxyMethyl)-7H-pyrrolo[2,3-d]pyriMidin-4-yl]-,(βR)-](/CAS/20180808/GIF/1236033-03-6.gif)
1236033-03-6
30 suppliers
inquiry