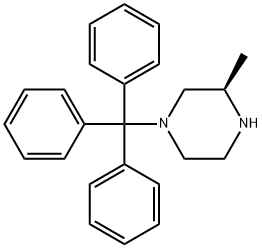
(R)-4-N-TRITYL-2-METHYL PIPERAZINE synthesis
- Product Name:(R)-4-N-TRITYL-2-METHYL PIPERAZINE
- CAS Number:313657-75-9
- Molecular formula:C24H26N2
- Molecular Weight:342.48
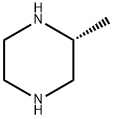
75336-86-6
235 suppliers
$9.00/5g
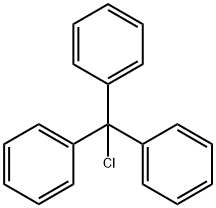
76-83-5
702 suppliers
$6.00/25g

313657-75-9
45 suppliers
$51.20/250mg
Yield:313657-75-9 100%
Reaction Conditions:
with triethylamine in dichloromethane;acetonitrile at 20 - 30; for 1.5 h;
Steps:
6 EXAMPLE 6-Preparation [ OF (R)-3-METHYL-L-TRITYLPIPERAZINE]
To the 1200 L reactor was dissolved (R)-2-methylpiperazine from Example 5 (25 kg) in CH3CN (319 kg) at [15°C] to [25°C] until dissolution was complete (10 min. ) After cooling to [5°C] to [10°C] Et3N (63 kg) was added. To the 1200 L receiver trityl chloride (69.5 kg) was dissolved in CH2C12 (106 kg) at [15°C] to [25°C.] The solution in the receiver was transferred to the reactor over 0.5 h with a CH2C12 rinse (27 kg), and the solution was heated to [20°C] to [30°C.] The reaction was monitored by GLC and was complete in 1 h. The resulting slurry was cooled to [8°C] to [12°C,] filtered onto a 48"Nutsche filter, and rinsed with CH3CN (40 kg at [8°C] to [12°C).] The filter cake was dried using [50°C] to [55°C] nitrogen to afford 25.26 kg of the by-product [ET3NHCL] (74% yield; easy to filter off the by-product). The filtrate was transferred to the 1200 L reactor and cooled further [TO-8°C] [TO-10°C] for 1 h. The resulting slurry was filtered onto a 24"Nutsche filter and rinsed [WITH-8°C] to-10°C CH3CN (24 kg) sending the filtrate and rinse to the 1200 L receiver. The filter cake was dried with [50°C] to [55°C] nitrogen to afford another 2.98 kg [ET3NHCL] (9% yield). The filtrate was transferred to the 1200 L reactor with a CH3CN (10 kg) rinse, and distilled under vacuum to an oil of the title compound of 97.99% GC purity. The yield was quantitative. M. Pt. [134-136°C.] ['H] NMR (400 MHz, CDC13) : [6] 7.55-7. 40 (6H, br s), 7.25 (6H, t, J = 7. 9 Hz), 7.14 (3H, t, [J =] 7.1 Hz), 3.21-3. 13 (2H, [M),] 3. [10-2. 90 (1H,] br s), 2.94 (2H, t, J = 13.0 Hz), 1.60 [(1H,] br s), 1.48 (1H, br s), 1.15 (1H, br s), 0.94 (3H, d, J = 6.1 Hz), 0.00 (TMS, reference). [13C] NMR (100 MHz, CDC13) : [6] 129.41 (d), 127.46 (d), 125.96 (d), 125.83 (s), 56.12 (t), 51.23 (d), 48.73 (t), 46.45 (t), 20.05 (q), 0.00 (TMS, reference). IR (diffuse reflectance) 2964,2835, 2483 (w), 2350 (w), 2339 (w), 1956 (w), 1490,1025, 909,742 (s), 717,710 (s), 703 (s), 697 (s), 629, [CM-1.] HRMS [(EI)] calcd for [C24H26N2] 342.2096, found [342. 2101.] [a] [25D=-12° (C 1. 00, CH2CL2).] Anal. Calcd for [C24H26N2] : C, 84.17 ; H, 7.65 ; N, 8.18. Found: C, 84.12 ; H, 7.64 ; N, 7.94.
References:
WO2004/829,2003,A1 Location in patent:Page 26
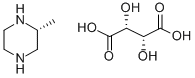
126458-16-0
16 suppliers
inquiry

313657-75-9
45 suppliers
$51.20/250mg

109-07-9
371 suppliers
$5.00/5g

313657-75-9
45 suppliers
$51.20/250mg