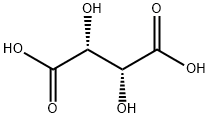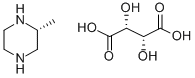
(R)-2-METHYL PIPERAZINE (L)TARTARIC ACID SALT synthesis
- Product Name:(R)-2-METHYL PIPERAZINE (L)TARTARIC ACID SALT
- CAS Number:126458-16-0
- Molecular formula:C9H18N2O6
- Molecular Weight:250.25
Yield:126458-16-0 74%
Reaction Conditions:
in ethanol;water at 10 - 22; for 2 h;
Steps:
4 EXAMPLE 4-Preparation of [(R)-2-METHYLPIPERAZINE,] L-tartrate
To the 1200 L reactor was added [60°C] racemic 2-methylpiperazine (100 kg) from a drum. H20 (240 L) was added, and the solution was cooled to [13°C.] To the 1200 L receiver was added L-tartaric acid (150 kg). [H20] (140 L) was added, and the slurry was stirred for 1 h 35 min until dissolution of the solids was complete. The L-tartaric acid solution was transferred to the 1200 L reactor over 2 h while maintaining a temperature of [10-22°C] in the 1200 L reactor followed by a [H20] rinse (20 L). Ethanol (163 kg) was added to the 1200 L reactor, and the solution was cooled to [2°C.] The resulting slurry was stirred for 2 h at [2°C,] and filtered through a 36"Nutsche filter sending the filtrate into the 1200 L receiver. The 1200 L reactor and 36"Nutsche filter were washed with H20 (200 L), and the solids were dried to yield 214 kg of 12% ee [(171%] based on the title compound). These solids were recharged to a clean 1200 L receiver and H20 (630 L) was added to the 1200 L receiver, which was heated to [85°C] until all the solids had dissolved. The solution was filtered through an in-line filter (C) into the 1200 L reactor, cooled to [5°C,] and stirred for 2 h. The resulting slurry was filtered through a clean 36"Nutsche filter sending the filtrate into the 1200 L receiver. The 1200 L reactor and 36"Nutsche filter were washed with [H20] (200 L), and the solids were dried to yield 104 kg of 93% ee (83% based on the title compound). These solids were recharged to a clean 1200 L receiver and [H20] (254 L) was added to the 1200 L receiver, which was heated to [85°C] until all the solids had dissolved. The solution was filtered through an in-line filter (C) into the 1200 L reactor, cooled to [5°C,] and stirred for 2 h. The resulting slurry was filtered through a clean 36"Nutsche filter sending the filtrate into the 1200 L receiver. The 1200 L reactor and 36"Nutsche filter were washed with H20 (200 L), and the solids were dried to yield 92 kg of 99% ee (74% based on the title compound). [D1] does not describe the preparation of the title compound but makes reference [TO J.] Med. Chem. 1990, 33, 1645-1656 (D2). The yield of the title compound according to D2, starting from racemic 2-methylpiperazine was 35%. M. Pt. 255.0-257. [0°C.] 'H NMR (400 MHz, [D20)] : [6] 4.79 [(D20,] reference), 4.36 (2H, s), 3.73-3. 64 (4H, [M),] 3.43 [(1H,] td J = 13.7, 3.0 Hz), 3.34 [(1H,] td, J = 12.7, 3.1 Hz), 3.17 [(1H,] dd, J = 14.2 12.8 Hz), 1.41 (3H, d, J = 6.1 Hz), 0.00 (TMS, reference). [13C] NMR (100 MHz, [D20)] : [6] 178.46 (s), 73.91 (d), 49.02 (d), 49.00 [(MEOH,] reference), 45.82 (t), 40.56 (t), 40.10 (t), 15.42 (q). IR (diffuse reflectance) 3426 (s), 3011 (s), 2999 (s), 2888 (s), 2785 (s, b), 2740 (s, b), 2703 (s, b), 2649 (s, b), 2483 (s, b), 2483 (s, b), 2361 (s), 2354,2340, 2248,1638 (s), cm HRMS (FAB) [CALCD FOR C5HL2N2 +HI 101.] 1079, found [101.] 1080. [[A]] [25D] = [24°] (c 1.00, water). Anal. Calcd for [C4H606.] [C5HL2N2C,] 43.20 ; H, 7.25 ; N, 11.19. Found: C, 41.25 ; H, 7.45 ; N, 10.71.
References:
WO2004/829,2003,A1 Location in patent:Page 24-25