
Pyridoxamine dihydrochloride synthesis
- Product Name:Pyridoxamine dihydrochloride
- CAS Number:524-36-7
- Molecular formula:C8H14Cl2N2O2
- Molecular Weight:241.11
https://patents.google.com/patent/CN101628892A/en
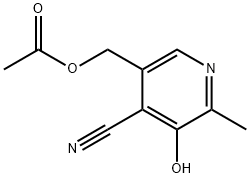
408335-89-7
2 suppliers
inquiry

524-36-7
315 suppliers
$9.00/1g
Yield:524-36-7 83%
Reaction Conditions:
with hydrogenchloride;hydrogen;5%-palladium/activated carbon in methanol;water at 20; under 760.051 Torr;
Steps:
6
Example 6; Production of 2-methyl-3-hydroxy-4-aminomethyl-5-hydroxymethyl-pyridine (pyridoxamine) dihydrochloride; 214 mg (1.04 mmol) of 2-methyl-3-hydroxy-4-cyano-5-acetyloxymethyl-pyridine were dissolved in 12 mol of methanol containing 300 μl of 30% hydrochloric acid at room temperature. 40 mg of 5 w/w% palladium on charcoal was added to the solution, and the mixture was hydrogenated under atmospheric pressure and at room temperature. The catalyst was then removed by filtration and the solvent of the filtrate removed under reduced pressure. The residue was recystallized from ethanol to afford 208 mg (0.86 mmol) of pyridoxamine dihydrochloride as an off-white solid; the amount obtained represented a 83% yield. The analytical data (1H and 13C NMR, LC, LC/MS and UV) obtained for the product confirmed through comparison of such data with those of a sample of commercially available pyridoxamine dihydrochloride that the product was the desired one.
References:
WO2006/66806,2006,A1 Location in patent:Page/Page column 16
![1H-Isoindole-1,3(2H)-dione, 2-[[3-(acetyloxy)-5-[(acetyloxy)methyl]-2-methyl-4-pyridinyl]methyl]-](/CAS/20210305/GIF/863030-57-3.gif)
863030-57-3
1 suppliers
inquiry

524-36-7
315 suppliers
$9.00/1g
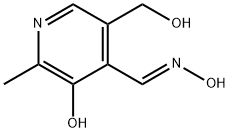
20905-66-2
1 suppliers
inquiry

524-36-7
315 suppliers
$9.00/1g
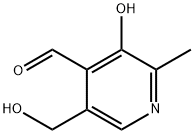
66-72-8
42 suppliers
inquiry

524-36-7
315 suppliers
$9.00/1g
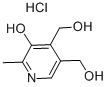
58-56-0
602 suppliers
$5.00/100mg

524-36-7
315 suppliers
$9.00/1g