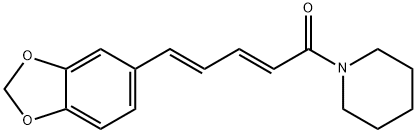
Piperine synthesis
- Product Name:Piperine
- CAS Number:94-62-2
- Molecular formula:C17H19NO3
- Molecular Weight:285.34

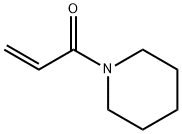
110-89-4
3 suppliers
$15.96/100ML
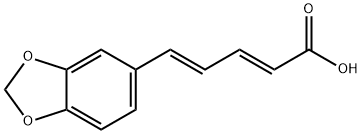
136-72-1
33 suppliers
$65.00/50mg

94-62-2
421 suppliers
$14.00/250mg
Yield:94-62-2 95%
Reaction Conditions:
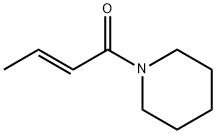
Stage #1: piperic acidwith thionyl chloride in dichloromethane at 20;Schlenk technique;
Stage #2: piperidine in dichloromethane at 20; for 1 h;Schlenk technique;Inert atmosphere;stereoselective reaction;
Steps:
General procedure: In a flame-dried Schlenk tube the carboxylic acid 4 (0,20 mmol,1.0 equiv.) was dissolved in anhydrous DCM (1 mL), followed bythe addition of thionyl chloride (0.40 mmol, 2.0 equiv.). Thereaction was stirred at room temperature until the solid dissolvedcompletely. Heating to reflux can be applied for a faster conversion. Thereafter, volatiles were removed under reducedpressure, and the flask was flushed with argon again (an argonfilledballoon was attached to the rotary evaporator). To theresulting residue anhydrous DCM was added (1 mL), and theamine was added dropwise (0.40 mmol, 2.0 equiv.). Afterwards,the solution was stirred for 1 h at room temperature. Finally, thereaction mixture was quenched and washed with saturatedaqueous NaHCO3 solution, and the aqueous layer was extracted3 times with DCM. The combined organic layer was dried overNa2SO4, filtered, and volatiles were removed under reducedpressure to yield the pure amide. Further purification via flashchromatography gave the desired amide. (Gradient: EtOAc/heptane10:90 to EtOAc/heptane 60:40).
References:
Bauer, Adriano;Nam, Jun-Hyun;Maulide, Nuno [Synlett,2019,vol. 30,# 4,p. 413 - 416]
![Ethanone, 2-[[(2E)-3-(1,3-benzodioxol-5-yl)-2-propen-1-yl]sulfonyl]-1-(1-piperidinyl)-](/CAS/20210305/GIF/736947-76-5.gif)
736947-76-5
0 suppliers
inquiry

94-62-2
421 suppliers
$14.00/250mg
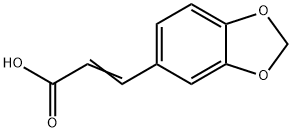
10043-37-5
12 suppliers
inquiry
2373-80-0
180 suppliers
$12.00/10g

94-62-2
421 suppliers
$14.00/250mg
![1,3-Benzodioxole, 5-[(1E)-2-iodoethenyl]-](/CAS/20210305/GIF/202998-56-9.gif)