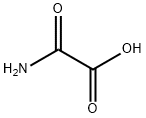
Oxamic acid synthesis
- Product Name:Oxamic acid
- CAS Number:471-47-6
- Molecular formula:C2H3NO3
- Molecular Weight:89.05
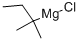
28276-08-6
31 suppliers
$426.30/100ml
139419-63-9
9 suppliers
$262.00/500mg

471-47-6
113 suppliers
$14.14/1gm:
186268-77-9
25 suppliers
$257.00/500mg
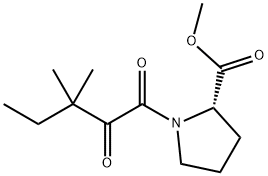
Yield:186268-77-9 2.10 g (75%)
Reaction Conditions:
in tetrahydrofuran;hexane;
Steps:
1.2 Step 2:
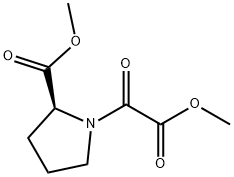
Step 2: Synthesis of methyl (2S)-1-(1,2-dioxo-3,3-dimethylpentyl)-2-pyrrolidinecarboxylate A solution of methyl (2S)-1-(1,2-dioxo-2-methoxyethyl)-2-pyrrolidinecarboxylate (2.35 g; 10.90 mmol) in 30 mL of tetrahydrofuran (THF) was cooled to -78° C. and treated with 14.2 mL of a 1.0 M solution of 1,1-dimethylpropylmagnesium chloride in THF. After stirring the resulting homogeneous mixture at -78° C. for three hours, the mixture was poured into saturated ammonium chloride (100·mL) and extracted into ethyl acetate. The organic phase was washed with water, dried, and concentrated, and the crude material obtained upon removal of the solvent was purified on a silica gel column, eluding with 25% ethyl acetate in hexane, to obtain 2.10 g (75%) of the oxamate as a colorless oil. 1H NMR (CDCl3): d 0.88 (t, 3H), 1.22, 1.26 (s, 3H each), 1.75 (dm, 2H), 1.87-2.10 (m, 3H), 25 2.23 (m, 1H), 3.54 (m, 2H), 3.76 (s, 3H), 4.52 (dm, 1H, J=8.4, 3.4).
References:
US2010/317711,2010,A1

95-92-1
417 suppliers
$5.00/5G

471-47-6
113 suppliers
$14.14/1gm:
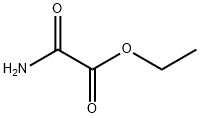
617-36-7
143 suppliers
$9.00/5g

471-47-6
113 suppliers
$14.14/1gm:
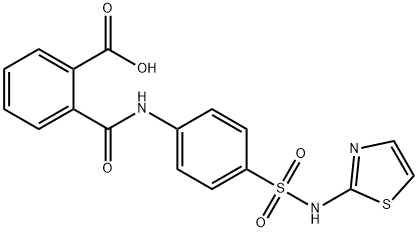
85-73-4
238 suppliers
$36.96/25G

471-47-6
113 suppliers
$14.14/1gm:

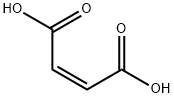
144-62-7
766 suppliers
$5.00/5g
110-16-7
724 suppliers
$10.00/5g
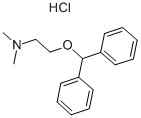
147-24-0
665 suppliers
$5.00/250mg

64-18-6
1084 suppliers
$22.12/250ML

471-47-6
113 suppliers
$14.14/1gm:

144-62-7
766 suppliers
$5.00/5g