
Naltrexone hydrochloride synthesis
- Product Name:Naltrexone hydrochloride
- CAS Number:16676-29-2
- Molecular formula:C20H24ClNO4
- Molecular Weight:377.87
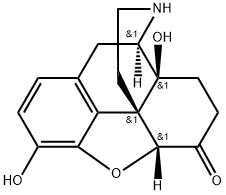
33522-95-1
87 suppliers
$103.00/1mg
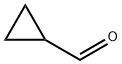
1489-69-6
315 suppliers
$6.00/1g

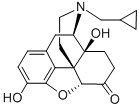
16676-29-2
285 suppliers
$21.00/1mg
Yield:16676-29-2 74%
Reaction Conditions:
Stage #1: noroxymorphone;Cyclopropanecarboxaldehydewith hydrogen;platinum on activated charcoal in 1-methyl-pyrrolidin-2-one;methanol at 50; under 2068.65 Torr; for 1 h;
Stage #2: with hydrogenchloride in ethanol;water; pH=< 4;Product distribution / selectivity;
Steps:
2
Example 2: Preparation of Naltrexone Hydrochloride from Noroxymorphone; Noroxymorphone alkaloid (20.Og, equivalent to 60.9mmol dry) was added to a mixture of iV-methylpyrrolidinone (60ml) and methanol (140ml). Cyclopropanecarboxaldehyde (5.3ml, 70.9mmol) and platinum on carbon catalyst were added and the mixture hydrogenated at 40psi and 50°C for 1 hour. Upon completion, the catalyst was filtered off and the reaction liquors diluted with chloroform (60ml) and washed with water (200ml). The aqueous layer was extracted with chloroform (2 x 60ml) and the combined organic layer washed with water (5 x 140ml). The organic layer was concentrated down to dryness and the solid residue redissolved in ethanol (100ml). Hydrochloric acid was added until pH < 4.0. The resulting precipitate was filtered, washed with ethanol (10ml) and dried in oven to yield a white solid. 16.7g (74% theory). 1H NMR (d6-DMSO, δ /ppm): 0.60 (IH, m), 0.70 (IH, m), 0.80 (2H, m), 1.30 (IH, m), 1.70 (2H, m), 2.20 (lH,m), 2.30 (lH,m), 2.65 (2H,m), 2.85 (lH,m), 3.15 (3H, br m), 3.50 (2H,m), 4.17 (IH, br d), 5.20 (lH,s), 6.80 (IH, d, J = 8Hz), 6.85 (IH, d, J = 8Hz), 7.18 (IH, s), 9.20 (IH, brs), and 9.70 (IH, s). 13C NMR (d6-DMSO δ /ppm): 2.72, 5.24, 5.81, 22.98, 27.18, 30.73, 35.16, 46.11, 48.66, 56.73, 60.82, 69.83, 88.64, 118.09, 119.83, 120.59, 127.89, 140.22, 143.54, and 207.94. HPLC and IR spectra were consistent against a Naltrexone reference standard.
References:
WO2006/35195,2006,A1 Location in patent:Page/Page column 7
16590-41-3
152 suppliers
$32.79/1mL

16676-29-2
285 suppliers
$21.00/1mg

76-41-5
2 suppliers
$18.00/1mg

16676-29-2
285 suppliers
$21.00/1mg
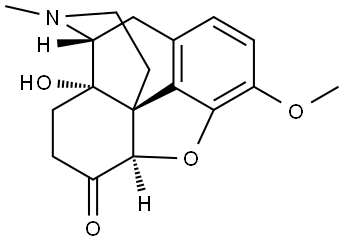
76-42-6
2 suppliers
$18.00/1mg

16676-29-2
285 suppliers
$21.00/1mg
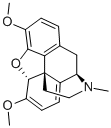
115-37-7
2 suppliers
$21.00/1mg

16676-29-2
285 suppliers
$21.00/1mg