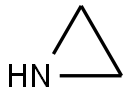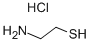
Cysteamine hydrochloride synthesis
- Product Name:Cysteamine hydrochloride
- CAS Number:156-57-0
- Molecular formula:C2H8ClNS
- Molecular Weight:113.61
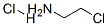
870-24-6
506 suppliers
$5.00/5g

156-57-0
612 suppliers
$5.00/5g
Yield:156-57-0 95%
Reaction Conditions:
Stage #1: 2-chloroethanamine hydrochloridewith sodium thiocarbonate in water at 40 - 60;
Stage #2: with hydrogenchloride in water at 70 - 85;
Steps:
2.2; 2.3; 2.4; 2.5
Step 2, 100 mL of a 2-chloroethylamine hydrochloride aqueous solution having a concentration of 1.01 mol / L was added dropwise to the above sodium thiocarbonate solution,During the dropping process, the temperature is controlled to 40-45 ° C, and the dropping time is 2h.After the dropwise addition, wait for 0.5h, then slowly increase the temperature to 60 ° C.And keep the temperature to continue the reaction,Until the color of the sodium thiocarbonate solution completely disappears, the reaction end point is reached;
Step 3, Continue to add 100 mL of hydrochloric acid with a concentration of 2.02 mol / L slowly to the mixture obtained after the previous reaction.During the dropwise addition, the temperature is controlled to 70 ° C, and the dropwise addition time is controlled to 2h.After the dropwise addition is completed, slowly raise the temperature to 85 ° C.The reaction is complete when no carbon disulfide is distilled off,To get a cysteamine hydrochloride mother liquor,After the carbon disulfide produced by the reaction is recovered through condensation, it can be reused as the reaction raw material in step 1.
Step 4, The solution obtained after the reaction in step 3 is completed is concentrated by distillation under reduced pressure until the solution volume is halved.Cooling down to precipitate sodium chloride crystals,The sodium chloride crystals were then removed by filtration,The remaining filtrate is the second stage mother liquor of cysteamine hydrochloride;
Step 5,Distill the second stage mother liquor of cysteamine hydrochloride obtained in step 4 under reduced pressure until the solution volume is halved.Cooling and crystallization to obtain 10.9 g of solid cysteamine hydrochloride,Based on sodium sulfide, the total mass yield was about 95.0%.
References:
CN110845375,2020,A Location in patent:Paragraph 0039; 0042-0045
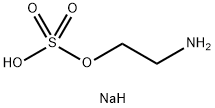
666-03-5
0 suppliers
inquiry

156-57-0
612 suppliers
$5.00/5g
![Ethanethioic acid, S-[2-[[(1,1-dimethylethoxy)carbonyl]amino]ethyl] ester (9CI)](/CAS/GIF/114326-10-2.gif)
114326-10-2
14 suppliers
$155.00/50mg

156-57-0
612 suppliers
$5.00/5g
96-53-7
313 suppliers
$6.00/10g

156-57-0
612 suppliers
$5.00/5g