
N-BOC-1,6-diaminohexane synthesis
- Product Name:N-BOC-1,6-diaminohexane
- CAS Number:51857-17-1
- Molecular formula:C11H24N2O2
- Molecular Weight:216.32
TLC Rf 0.51; 1H NMR (CDCL3) d 4.54 (bs, 1H; NH), 3.05 1 2.62 (m, 4H; NCH2), 1.41 (m, 9H; CH3), 1.29 (m, 8H; CH2).
Evaluation of aminoalkylmethacrylate nanoparticles as colloidal drug carrier systems. Part I: synthesis of monomers, dependence of the physical properties on the polymerization methods

129392-87-6
19 suppliers
inquiry

51857-17-1
169 suppliers
$10.00/1g
Yield:51857-17-1 97%
Reaction Conditions:
with hydrogen;palladium on activated charcoal at 20; under 3102.97 Torr; for 1 h;
Steps:
A [6-T-BUTYLOXYCARBAMOYLHEXYLAMINE] (4).
A solution of di-t-butyldicarbonate (18.6 g, [85.] 3 mmol) in dry DCM (100 [ML)] was added dropwise to a stirred solution of 6- aminohexanol (10.0 g, 85.3 mmol) in dry DCM (100 [ML)] at 20 [°C] and stirred for 16 h. The solution was washed with dilute aqueous [NA2C03] solution (100 mL), 0.1 M HC1 (100 mL), water (100 mL), brine (50 mL), dried and the solvent evaporated. The residue was dissolved in DCM (250 mL) and Et3N (15.5 mL, 111 mmol) added. A solution of methanesulfonyl chloride (7.3 mL, 94 mmol) was added dropwise and the mixture stirred at [20 °C] for 16 h. The solution was washed with saturated aqueous [KHC03] (100 mL), water (2 x 100 mL), brine (50 mL), dried, and the solvent evaporated. The residue was dissolved in DMF (100 [ML)] and NaN3 [(5.] 55 g, [85.] 3 mmol) added. The mixture was stirred at [100 °C] for 1 h, the solvent evaporated and the residue partitioned between EtOAc (200 mL) and water (200 mL). The organic fraction was washed with water (200 mL), brine (100 mL), dried and the solvent evaporated. The residue was purified by chromatography, eluting with 30% EtOAc/pet. ether, to give the [6-T-BUTYLOXYCARBAMOYLHEXYL] azide (17.5 g, 85%) as a colorless [OIL, 1H NMR 8] 4.53 (br s, 1 H, OCONH), 3.52 (t, J= 6.9 Hz, 2 H, [CH2N),] 3.11 (dt, [J=] 6.5, 6.4 Hz, 2 H, [CH2N),] 1.57-1. 63 (m, 2 H, [CH2),] 1.44-1. 52 (m, 11 H, CH2, C [(CH3)] 3), 1.30-1. 40 (m, 4 H, 2 x [CH2), 13C NMR 8] 156.0, 79.1, 51.3, 40.4, 29.9, 28.7, 28.4 (3), 26.4, 26.3. [A] mixture of azide (14.81 g, 61.1 mol) and Pd/C (0.5 g) in EtOAc/EtOH (200 mL) was stirred at [20 °C] under hydrogen (60 psi) for 1 h. The mixture was filtered through celite, the cake washed with EtOAc [(3 X 30] mL) and the solvent evaporated to give 4 (12.82 g, [97%)] as a white solid, mp (EtOAc) [89-91 °C] ; 1H NMR 8 4.65 (br s, 1 H, OCONH), 3.52 (br s, 2 H, NH2), 2.69 (t, [J=] 6.9 Hz, 2 H, CH2N), 1. 88 (br s, 2 H, CH2N), 1.44-1. 50 [m, 13 H, 2 x [CHA,] C (CH3) 3], 1.29-1. 35 (m, 4 H, 2 x [CHEZ) ; 13C] NMR 8 156.0, 78.9, 41.9, 40.4, 33.4, 29.9, 28.3 (3), 26.5, 26.4.
References:
AUCKLAND UNISERVICES LIMITED;THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIVERSITY WO2004/26846, 2004, A1 Location in patent:Page 54

124-09-4
354 suppliers
$14.00/5g
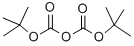
24424-99-5
832 suppliers
$13.50/25G

51857-17-1
169 suppliers
$10.00/1g

124-09-4
354 suppliers
$14.00/5g

6627-89-0
146 suppliers
$8.00/5g

51857-17-1
169 suppliers
$10.00/1g

124-09-4
354 suppliers
$14.00/5g

24424-99-5
832 suppliers
$13.50/25G

51857-17-1
169 suppliers
$10.00/1g

16644-54-5
11 suppliers
$125.00/500 mg

118110-05-7
5 suppliers
inquiry

51857-17-1
169 suppliers
$10.00/1g