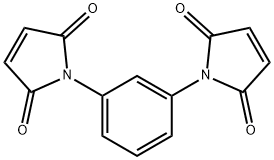
N,N'-1,3-Phenylene bismaleimide synthesis
- Product Name:N,N'-1,3-Phenylene bismaleimide
- CAS Number:3006-93-7
- Molecular formula:C14H8N2O4
- Molecular Weight:268.22

108-31-6
848 suppliers
$13.47/50 G
16674-78-5
396 suppliers
$6.00/100g

3006-93-7
263 suppliers
$20.00/25 g
Yield:3006-93-7 84%
Reaction Conditions:
with acetic anhydride in water;acetone
Steps:
1 EXAMPLE 1
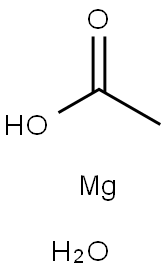
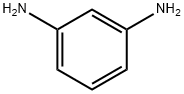
EXAMPLE 1 This example illustrates various metal salt co-catalysts that may be used to prepare N,N'-m-phenylenedimaleimide. A glass flask was equipped with a thermometer, mechanical paddle stirrer and a water cooled condenser. Crushed maleic anhydride (1.92 parts) was added to a solution of 1 part m-phenylenediamine and 13 parts acetone over a 15-minute period. The heterogeneous reaction mixture was stirred at 40° C. for 1 hour. Acetic anhydride (2.44 parts), 0.32 part triethylamine, and 0.04 part magnesium acetate tetrahydrate were added all at once. The temperature was raised to 60° C. using a heating mantle and maintained for 4 hours. The reaction mixture was cooled, 20 parts water added, and the precipitated product isolated by filtration using a vacuum filter funnel. The filter cake was washed with water and dried for 24 hours at 60°-70° C. in a vacuum oven. A 74% yield of N,N'-m-phenylenedimaleimide, m.p. 203°-204° C. was obtained. The example was repeated except that 0.04 part lithium acetate dihydrate was used as the catalyst instead of magnesium acetate tetrahydrate. N,N'-m-phenylenedimaleimide, m.p. 208° C., was obtained in 84% yield. The example was repeated except that 0.04 part manganous acetate tetrahydrate was used as catalyst instead of magnesium acetate tetrahydrate. N,N'-m-phenylenedimaleimide, m.p. 203°-204° C., was obtained in 80% yield. The example was repeated except that 0.02 part lithium chloride was used as catalyst instead of magnesium acetate tetrahydrate. N,N'-m-phenylenedimaleimide, m.p. 199° C., was obtained in 84% yield.
References:
E. I. Du Pont de Nemours and Company US4154737, 1979, A

108-31-6
848 suppliers
$13.47/50 G
108-45-2
457 suppliers
$13.00/25g

3006-93-7
263 suppliers
$20.00/25 g