
N-Boc-4-oxo-L-proline synthesis
- Product Name:N-Boc-4-oxo-L-proline
- CAS Number:84348-37-8
- Molecular formula:C10H15NO5
- Molecular Weight:229.23
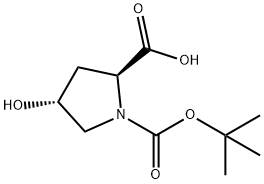
13726-69-7
435 suppliers
$5.00/1g

84348-37-8
283 suppliers
$5.00/250mg
Yield:84348-37-8 95.9%
Reaction Conditions:
with 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;trichloroisocyanuric acid in ethyl acetate at -5 - 30; for 1.33333 h;
Steps:
2 Example 2: Preparation of 1-(tert-butoxycarbonyl)-4-oxo-L-proline (Formula V)
Trichloroisocyanuric acid (75.6 g) was added to a solution of (4R)-1-(tert- butoxycarbonyl)-4-hydroxy-L-proline (Formula IV, prepared according to the process of Example 1; 100 g) in ethyl acetate (1000 mL). The solution was cooled to 0°C to -5°C. A solution of TEMPO in ethyl acetate (3.38 g in 50 mL ethyl acetate) was slowly added to the reaction mixture at -5°C to 10°C, and the reaction mixture was stirred at the same temperature for 20 minutes. The reaction mixture was heated to a temperature of 25°C to 30°C, and stirred at the same temperature for 60 minutes. After completion of the reaction, the reaction mixture was quenched with deionized water (200 mL), and stirred for 60 minutes at a temperature of about 25°C to 30°C. The reaction mixture was filtered through a Hyflo bed. The filtrate was washed with deionized water (2 x 200 mL). The layers were separated. The organic layer was washed with an aqueous solution of sodium chloride (prepared by adding 40 g sodium hydroxide to 200 mL deionized water). The organic layer was concentrated at a temperature of about 50°C under reduced pressure to obtain a residue. The residue was dissolved in ethyl acetate ( 100 mL). Hexanes (400 mL) were slowly added to the solution of ethyl acetate. The reaction mixture was stirred at 25°C to 30°C for 30 minutes. The reaction mixture was filtered to obtain a solid. The solid was washed with a mixture of ethyl acetate (20 mL) and hexanes (80 mL), and dried at 40°C to 45°C under reduced pressure to obtain 1-(tert-butoxycarbonyl)-4-oxo-L-proline. Yield: 95.9%
References:
WO2015/19238,2015,A1 Location in patent:Page/Page column 9
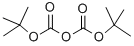
24424-99-5
839 suppliers
$13.50/25G

51-35-4
855 suppliers
$6.00/5g

84348-37-8
283 suppliers
$5.00/250mg
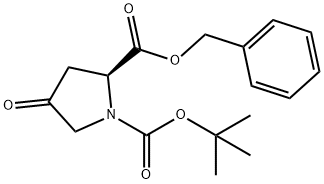
154456-97-0
35 suppliers
inquiry

84348-37-8
283 suppliers
$5.00/250mg

7664-93-9
6 suppliers
$23.30/1090731000

13726-69-7
435 suppliers
$5.00/1g

84348-37-8
283 suppliers
$5.00/250mg

147266-92-0
174 suppliers
$60.00/100mg

84348-37-8
283 suppliers
$5.00/250mg