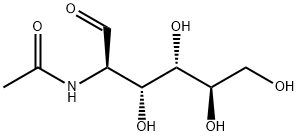
N-Acetyl-D-Glucosamine synthesis
- Product Name:N-Acetyl-D-Glucosamine
- CAS Number:7512-17-6
- Molecular formula:C8H15NO6
- Molecular Weight:221.21
Yield:-
Reaction Conditions:
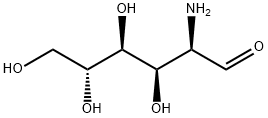
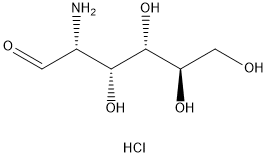
Stage #1: D-(+)-glucosamine hydrochloride in water;Industry scale;
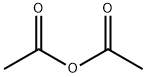
Stage #2: acetic anhydridewith sodium carbonate in water at 20 - 25; pH=4 - 6;Product distribution / selectivity;Industry scale;
Steps:
2
In two separate columns, 40 L weakly basic anion exchanger Indion-860 resin (product of Ion Exchange India Ltd, India) capacity 1.7 mEq/ml and 25 L of weakly acidic cation exchanger Indion-236 resin (product of Ion Exchange India Ltd, India) capacity 4.1 mEq/ml, were packed and regenerated as per known process of prior art.Glucosamine hydrochloride (GLH) 5.0 kg was dissolved in 20.0L (4 parts) of purified water at 30-35° C. Resultant clear solution was passed through anion exchanger column at a rate of 40 L (one bed volume) per hour. Initial void volume (15 L) comprising of water was discarded. The product stream with glucosamine base in solution was detected by change in refractive index and was collected in reactor, along with 20 L (0.5 bed volume) of purified water washing passed though the column. The total volume including washing was 43 L.Glucosamine base content in solution was estimated by colorimetric method described above. The yield of Glucosamine base was 3.3 kg (80%). The glucosamine base solution was cooled to 20-25° C. Acetic anhydride (2.7 kg) was added drop wise over period of 60 minutes and temperature of exothermic reaction was maintained at 20-25° C. The reaction pH was continuously controlled in the range of 4 to 6 by addition of 25% Sodium carbonate solution. Acetic anhydride addition was completed in 60 minutes and stirring was continued for further 15 minutes.The reaction solution was then passed through weakly acidic cation exchanger column at feed rate of 36 L (1.4 bed volume) per hour. Initial void volume (9.0 L) comprising of water was discarded. The product stream with N-acetyl-D-glucosamine in solution was detected by change in refractive index and was collected along with 12 L (0.5 bed volume) of purified water washing passed through column. NAG solution was then concentrated by distillation under vacuum (0.1 mm Hg) at temperature below 35° C. The crude NAG (5.8 Kg) with 30% moisture content was dissolved in 12.0 L of purified water and solution was decolorized by activated charcoal treatment. Decolorized and filtered NAG solution was concentrated again to less than 30% moisture content. The NAG crystals were then suspended in two parts by weight of methanol and stirred for 30 minutes at temperature below 10° C. and then kept at same temperature for 6 hours. Filtered NAG crystals were further washed with chilled methanol and then dried under vacuum at 50° C. The yield of N-acetyl-D-Glucosamine was 3.5 kg (68%), with melting point of 202-204° C. and material showed no darkening on incubation at 105° C. for 2 hours.
References:
US2012/264929,2012,A1 Location in patent:Page/Page column 3
10139-01-2
71 suppliers
$60.00/50mg

7512-17-6
617 suppliers
$5.00/1g
66-84-2
773 suppliers
$6.00/25g

7512-17-6
617 suppliers
$5.00/1g