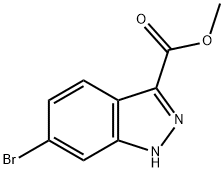
METHYL 6-BROMO-1H-INDAZOLE-3-CARBOXYLATE synthesis
- Product Name:METHYL 6-BROMO-1H-INDAZOLE-3-CARBOXYLATE
- CAS Number:885278-42-2
- Molecular formula:C9H7BrN2O2
- Molecular Weight:255.07
67-56-1
757 suppliers
$9.00/25ml
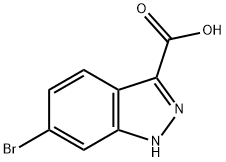
660823-36-9
142 suppliers
$31.00/100mg

885278-42-2
103 suppliers
$73.00/250mg
Yield:885278-42-2 76%
Reaction Conditions:
with sulfuric acid at 90; for 4 h;
Steps:
A
To a suspension of 6-bromo-1H-indazole-3-carboxylic acid (3.00 g, 12 mmol) in methanol (50 mL, 1 mol) was added Sulfuric acid (1.50 mL, 28 mmol), and the mixture was heated to 90 °C for 4 hours. After cooling to rt, the mixture was diluted with 200 mL EtOAc, and washed with 150 mL sat. NaHC0 2 (aq) and 150 mL brine. The organic extracts were dried (Na 2 S0 4 ) and concentrated in vacuo to provide 2.42 g (76%) of pure 6-bromo-lH-indazole-3-carboxylic acid methyl ester as a yellow solid. This material was diluted in tetrahydrofuran (50 mL), cooled to 0 °C, then sodium hydride (0.417 g, 10.4 mmol) was added. The mixture was stirred at 0 °C for 30 minutes, then [β-(trimethylsilyl)ethoxy]methyl chloride (1.85 mL, 10.4 mmol) was added dropwise. The mixture was allowed to slowly warm to room temperature over 90 minutes, then MeOH was added to quench excess hydride, and the mixture was concentrated in vacuo. The residue was diluted with 200 mL EtOAc, then washed with 200 mL brine. The aqueous layer was further extracted with 50 mL EtOAc, then the combined organic extracts were dried (Na2SO4 ) and concentrated in vacuo. Purification by CombiFlash (40 g column; load with CH2Cl2 ; 100:0 to 50:50 heptane:EtOAc over 30 minutes) provided 3.22 g (88%) of the title compound as a yellow oil.
References:
WO2013/24011,2013,A1 Location in patent:Page/Page column 51

660823-36-9
142 suppliers
$31.00/100mg

885278-42-2
103 suppliers
$73.00/250mg

6326-79-0
276 suppliers
$6.00/1g

885278-42-2
103 suppliers
$73.00/250mg
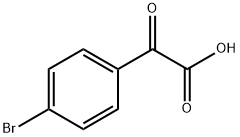
7099-87-8
53 suppliers
$35.00/100mg

885278-42-2
103 suppliers
$73.00/250mg