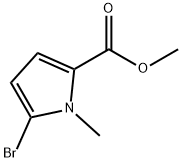
Methyl 5-broMo-1-Methyl-1H-pyrrole-2-carboxylate synthesis
- Product Name:Methyl 5-broMo-1-Methyl-1H-pyrrole-2-carboxylate
- CAS Number:1196-07-2
- Molecular formula:C7H8BrNO2
- Molecular Weight:218.05
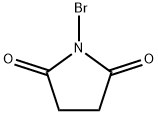
128-08-5
841 suppliers
$5.00/5 g

37619-24-2
105 suppliers
$10.00/500mg

1196-07-2
37 suppliers
$175.00/100mg
Yield: 64%
Reaction Conditions:
in dichloromethane;nitrogen
Steps:
34.a Step 34a
Step 34a To a dry flask is added methyl 1-methyl-1H-pyrrole-2-carboxylate (12.0 g, 86.4 mmol) and 150 mL of dry CH2Cl2, and the flask is wrapped in foil and purged with nitrogen. N-Bromosuccinimide (16.2 g, 90.7 mmol) is added in one portion and the mixture is stirred at rt for 0.5 h. The reaction mixture is washed with water (50 mL) and brine (50 mL), dried over MgSO4, filtered, and concentrated under reduced pressure. Fractional distillation gives 12.0 g of methyl 5-bromo-1-methyl-1H-pyrrole-2-carboxylate as a yellow oil (64% yield). MS for C7H8NO2Br (ESI) (M)+m/z 217.1.
References:
Piotrowski, David W.;Myers, Jason K.;Rogers, Bruce N.;Jacobsen, Eric Jon;Bodnar, Alice L.;Groppi JR., Vincent E.;Walker, Daniel Patrick;Acker, Brad A. US2003/207913, 2003, A1

37619-24-2
105 suppliers
$10.00/500mg

1196-07-2
37 suppliers
$175.00/100mg