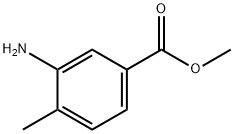
Methyl 3-amino-4-methylbenzoate synthesis
- Product Name:Methyl 3-amino-4-methylbenzoate
- CAS Number:18595-18-1
- Molecular formula:C9H11NO2
- Molecular Weight:165.19
67-56-1
756 suppliers
$9.00/25ml
2458-12-0
337 suppliers
$15.00/5g

18595-18-1
359 suppliers
$8.00/10g
Yield:18595-18-1 97%
Reaction Conditions:
with dichloro sulfoxide in methanol; for 4 h;Cooling with ice;
Steps:
1.2. Methyl 3-amino-4-methylbenzoate (2)
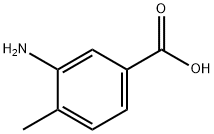
In a round bottom flask were added 3-amino-4-methylbenzoic acid 1 (1.00 g, 6.62 mmol) and anhydrous MeOH (25 mL). The solution was cooled in an ice bath and SOCl2 (1.71 g, 14.4 mmol, 1.05 mL) was added dropwise. The solution was stirred and refluxed for 4 h and then concentrated under reduced pressure. A saturated aqueous NaHCO3 solution (40 mL) was added and the mixture was extracted with EtOAc. Combined organic phases were dried over MgSO4. After evaporation, compound 2 (1.06 g, 6.42 mmol, 97%) was obtained as a beige powder.
References:
Auvert, Etienne;Aesoy, Reidun;Giraud, Francis;Herfindal, Lars;Anizon, Fabrice;Moreau, Pascale [Bioorganic and Medicinal Chemistry Letters,2022,vol. 73,art. no. 128914]
7356-11-8
174 suppliers
$6.00/10g

18595-18-1
359 suppliers
$8.00/10g
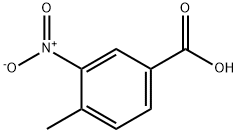
96-98-0
357 suppliers
$8.00/10g

18595-18-1
359 suppliers
$8.00/10g

19438-10-9
247 suppliers
$6.00/5g

18595-18-1
359 suppliers
$8.00/10g
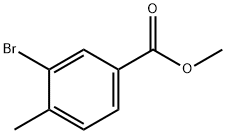
104901-43-1
204 suppliers
$6.00/1g

18595-18-1
359 suppliers
$8.00/10g