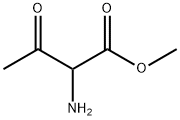
Methyl 2-amino-3-oxobutanoate synthesis
- Product Name:Methyl 2-amino-3-oxobutanoate
- CAS Number:68277-01-0
- Molecular formula:C5H9NO3
- Molecular Weight:131.13
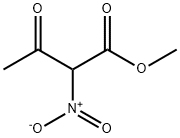
29291-62-1
0 suppliers
inquiry

68277-01-0
37 suppliers
inquiry
Yield:-
Reaction Conditions:
with nitric acid;acetic anhydride;zinc at 80; pH=3;Reflux;Concentration;
Steps:
1
First, a 500 mL three-necked flask equipped with a magnetic stirrer, a thermometer and a reflux condenser was charged with 30 g ofAcetoacetate and 50 mL of anhydrous acetic acid, the flask was placed in an ice bath at -5 ° C, stirred, stirred30min, then 50mL mass concentration of 0.3mol / L sodium nitrite solution in the bottle dropping, controlWas added dropwise over 25 min keeping the temperature of the flask within 8 & lt; 0 & gt; C during the dropwise addition, after which the reaction mixture was stirred at & lt;And the mixture was stirred at 8 ° C for 2 hours. After the reaction was completed, 18 g of acetic anhydride and 20 g of zinc powder were added to the bottle,The temperature of the water bath was raised to 80 ° C, and reflux was maintained at the same temperature. After refluxing, the mixture was quenched with 60 mass%Nitric acid adjust the solution pH value of 3, continue stirring reaction 2h; the reaction is completed, the reaction mixture in the bottleWas poured into a 500 mL Erlenmeyer flask and immediately 200 mL of ice water was added to the Erlenmeyer flask and stirred for 3 minAfter filtration, the filtrate was retained, and the resulting residue was washed three times with methylene chloride solution to remove unreacted impurities,The filtrate was distilled at 70 ° C to remove the solvent and the remaining solid was recrystallized from petroleum ether to give a solidBody, and mixed with the filter residue, the mixture into the oven at 65 for 40min, that 2,5-diacetylDimethylpyrrole; 15 g of the product obtained in the above reaction was dissolved in 200 g of anhydrous glacial acetic acid, after whichTo the reaction system, 30mL 0.2mol / L sulfuryl chloride was added dropwise,The temperature of the reaction was raised to 80C, refluxed for 1 h at this temperature, refluxed to room temperature, and the reactionThe resulting mixture was poured into a 500 mL Erlenmeyer flask and immediately 250 mL of ice water was added to the Erlenmeyer flask,The precipitate was washed once with distilled water, and then placed in an oven at 80 ° CIn dryness for 60 min to give 2-formyl-3,4-dimethyl-5-acetylpyrrole as a pale orange solid.
References:
CN105418476,2016,A Location in patent:Paragraph 0007
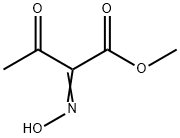
73708-95-9
5 suppliers
inquiry

68277-01-0
37 suppliers
inquiry

105-45-3
666 suppliers
$5.00/5g

68277-01-0
37 suppliers
inquiry
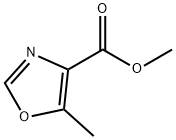
41172-57-0
83 suppliers
$80.00/500mg

68277-01-0
37 suppliers
inquiry
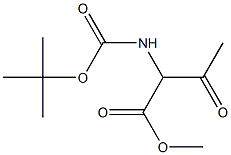
124576-57-4
2 suppliers
inquiry

68277-01-0
37 suppliers
inquiry