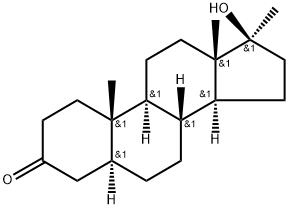
Mestanolone synthesis
- Product Name:Mestanolone
- CAS Number:521-11-9
- Molecular formula:C20H32O2
- Molecular Weight:304.47
Yield:521-11-9 20.6 g
Reaction Conditions:
Stage #1: methyl bromidewith iodine;magnesium in 2-methyltetrahydrofuran at 40 - 45; for 1 h;
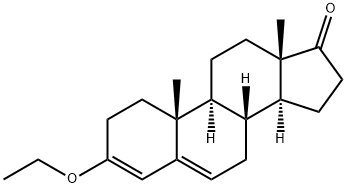
Stage #2: 3-ethoxyandrosta-3,5-dien-17-one in 2-methyltetrahydrofuran at 10 - 45; for 10.08 h;Further stages;
Steps:
2.2; 2.3 2. Preparation of Grignard
Add 560ml to the reaction flask2-methyltetrahydrofuran, 62.1g of magnesium turnings, 0.2g of iodine granules, stirred and heated to 40 ° C, began to slowly pass at 40 ° C ~ 45 ° CMethyl bromideUntil the magnesium flakes completely disappeared, continue to react at 40 ° C ~ 45 ° C for 1 hour, to obtain Grignard reagent, cooled to 10 ° C or less;103.5 g of the compound 3-ethoxy-androst-3,5-dien-17-one obtained in the previous reaction was added to 300 ml of 2-methyltetrahydrofuran, and stirred for 5 minutes to dissolve.a solution of 3-ethoxy-androst-3,5-dien-17-one in 2-methyltetrahydrofuran is added dropwise to the above-mentioned format reagent.The dropping temperature was controlled to be 10 ° C to 15 ° C.After the completion of the dropwise addition, the addition reaction is continued at 40 ° C to 45 ° C for 10 hours, the reaction is completed, concentrated at atmospheric pressure, and the residual paste obtained by concentration is cooled, and the concentrated residual paste is added to the previo3. Preparation of the compound male -17α-methyl-17β-hydroxy-3-oneAdd 30g of Grignard, 600ml of methanol, 150ml of dichloromethane to the reaction flask, stir evenly, add the alcohol solution of sodium hydroxide to adjust the pH to 8.5 ~ 9.0, add 9g of palladium on the palladium content of 2%, with hydrogen The air in the reaction bottle is replaced, and the hydrogen reaction is carried out at 25 ° C to 30 ° C for 9 hours;After the reaction is completed, the hydrogen in the reaction flask is replaced with nitrogen, and the solution of the hydride is obtained by filtration;Add 15g mass% concentration ≥35.0% purified hydrochloric acid to the hydride solution under stirring at room temperature, and carry out heat preservation hydrolysis reaction at 35 °C ~ 40 °C for 3 hours; after the reaction is completed, cool down to below 5 °C, add mass percentage concentration Adjust the pH to 6.0-7.0 with 5% aqueous sodium hydroxide solution, and evaporate the solvent under reduced pressure, then cool to below 5 °C.Slowly add 800ml of pure water, continue to cool down to below 5 °C, stir for 30 minutes, let stand for more than 2 hours, suction filtration, water washing,Dangxiong-17α-methyl-17β-hydroxy-3-one crude wet material.The crude product was recrystallized from industrial ethanol to give 20.6 g of andros- 17-methyl-17β-hydroxy-3-ketone, HPLC purity 99.3%.usly cooled to 0 ° C. The water of ~5 ° C was stirred for 2 hours, and allowed to stand for 2 hours or more, filtered, washed with water, and dried to obtain 117.6 g of Grignard.
References:
CN109627273,2019,A Location in patent:Paragraph 0020; 0022; 0023

74-87-3
210 suppliers
$18.50/48622
972-46-3
37 suppliers
inquiry

521-11-9
224 suppliers
$120.00/0.500g

972-46-3
37 suppliers
inquiry

74-88-4
350 suppliers
$15.00/10g

521-11-9
224 suppliers
$120.00/0.500g
75245-99-7
0 suppliers
inquiry

521-11-9
224 suppliers
$120.00/0.500g
641-82-7
12 suppliers
inquiry

521-11-9
224 suppliers
$120.00/0.500g