
4-Hydroxybenzaldehyde synthesis
- Product Name:4-Hydroxybenzaldehyde
- CAS Number:123-08-0
- Molecular formula:C7H6O2
- Molecular Weight:122.12
![Methanediol,1-[4-(acetyloxy)phenyl]-, 1,1-diacetate](/CAS/20180528/GIF/7143-16-0.gif)
7143-16-0
0 suppliers
inquiry

123-08-0
933 suppliers
$5.00/10g
Yield:123-08-0 96%
Reaction Conditions:
with ethanol at 50 - 60; for 0.0833333 h;
Steps:
2.3. General procedure for the deprotection of 1,1-diacetates
General procedure: A mixture of the 1,1-diacetate of the aldehyde (5 mmol) andP(4-VPH)ClO4 (20 mg) in ethanol (5 mL) was stirred at 50-60°C. Upon completion of the reaction (as determined by TLC),the mixture was filtered to remove the catalyst. The filtrate wascollected and distilled to dryness to give a residue, which waspartitioned between water (10 mL) and Et2O (5 mL). The etherlayer was collected and the aqueous layer was back-extractedwith Et2O (2 × 5 mL). The combined ether layers were washedwith a saturated solution of NaHCO3 (5 mL) and dried overanhydrous Na2SO4. The solvent was removed under vacuum togive the crude product as a residue, which was purified by silicagel column chromatography (n-hexane:ethyl acetate = 85:15(v/v)) to afford the pure aldehyde.
References:
Khaligh, Nader Ghaffari [Cuihua Xuebao/Chinese Journal of Catalysis,2014,vol. 35,# 3,p. 329 - 334]
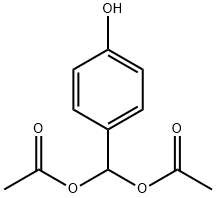
176443-11-1
0 suppliers
inquiry

123-08-0
933 suppliers
$5.00/10g
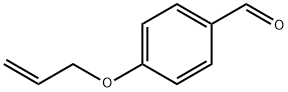
40663-68-1
120 suppliers
$10.00/1g

123-08-0
933 suppliers
$5.00/10g
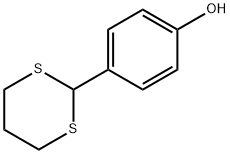
57529-05-2
12 suppliers
inquiry

123-08-0
933 suppliers
$5.00/10g
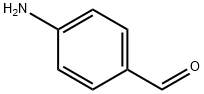
556-18-3
194 suppliers
$89.00/5g

123-08-0
933 suppliers
$5.00/10g