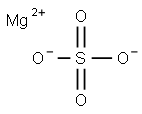
Magnesium sulfate synthesis
- Product Name:Magnesium sulfate
- CAS Number:7487-88-9
- Molecular formula:MgSO4
- Molecular Weight:120.37
MgO + H2SO4 → MgSO4 + H2O
Mg(OH)2 + H2SO4 → MgSO4 + 2H2O
MgCO3 + H2SO4 → MgSO4 + CO2 + H2O
Crystallization at temperatures between 1.8 and 48°C yields heptahydrate, MgSO4•7H2O. Below 1.8°C, a dodecahydrate , MgSO4•12H2O crystallizes out. Above 48°C crystals of lower hydrates form. The anhydrous salt is obtained by heating the heptahydrate at about 500°C in a rotary drum; or dehydrating above 150°C in the presence of sulfuric acid.

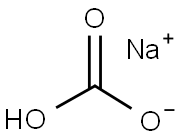
501-53-1
398 suppliers
$10.00/1g
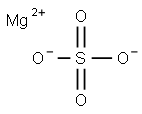
7487-88-9
675 suppliers
$5.00/25g
Yield:-
Reaction Conditions:
with sodium carbonate in 1,4-dioxane;water;
Steps:
2.2 2.
2. Preparation of Phosphonic Acid Intermediates: Amine I (9.0 g, 41.1 mmol) was dissolved in 1,4-dioxane (100 mL). A solution of Na2CO3 (13.1 g, 123.3 mmol) in H2O (50 mL) was added to the reaction mixture and stirred for 5 minutes at room temperature. After benzyl chloroformate (8.4 g, 49.3 mmol) was added, the reaction solution was stirred at room temperature overnight. The organic phase was diluted with EtOAc and extracted with H2O and brine. The organic phase was dried over MgSO4. Concentration of the filtrate from vacuum filtration removal of the MgSO4 yielded an oil from which II was isolated by column chromatography (SiO2, 20% EtOAc in hexane) as a clear oil (11.6 g, 80%). 1H NMR (300 MHz, CDCl3) δ 7.33 (s, 5H), 6.05 (dt, J=9.9, 17.1 Hz, 1H), 5.65 (d, J=23.7 Hz, 1H), 5. (d, J=17.1 Hz, 1H), 5.06 (m, 3H), 4.06 (m, 4H), 2.09 (m, 1H), 1.73 (m, 2H), 1.15 (dt, J=8.1, 26.4 Hz, 6H). 31P NMR (121.4 MHz, CDCl3) δ 23.7
References:
US2011/306541,2011,A1
![Magnesium, [sulfito(2-)-κO]- (9CI)](/CAS/20211123/GIF/791574-55-5.gif)
791574-55-5
0 suppliers
inquiry
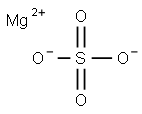
7487-88-9
675 suppliers
$5.00/25g