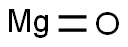
Magnesium oxide synthesis
- Product Name:Magnesium oxide
- CAS Number:1309-48-4
- Molecular formula:MgO
- Molecular Weight:40.3
MgO+H2O→Mg(OH)2
However, this reaction can be reversed by heating it to remove moisture.
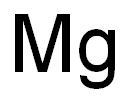
7439-95-4
8 suppliers
$16.00/0.9g
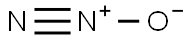
10024-97-2
1 suppliers
$436.00/227g
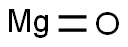
1309-48-4
692 suppliers
$5.00/100g
Yield:-
Reaction Conditions:
in gas;Mg was resistively heated to 700°C, and the metal vapor was entrained in an Ar carrier gas and allowed to react with N2O;;
References:
Azuma, Yoshiro;Dyke, Thomas R.;Gerke, Gretchen K.;Steimle, Timothy C. [Journal of Molecular Spectroscopy,1984,vol. 108,p. 137 - 142]
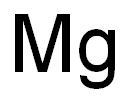
7439-95-4
8 suppliers
$16.00/0.9g
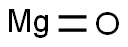
1309-48-4
692 suppliers
$5.00/100g
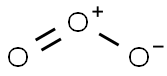
10028-15-6
22 suppliers
inquiry
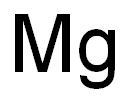
7439-95-4
8 suppliers
$16.00/0.9g
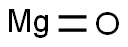
1309-48-4
692 suppliers
$5.00/100g