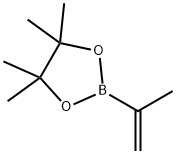
Isopropenylboronic acid pinacol ester synthesis
- Product Name:Isopropenylboronic acid pinacol ester
- CAS Number:126726-62-3
- Molecular formula:C9H17BO2
- Molecular Weight:168.04
76-09-5
385 suppliers
$8.19/250mg
557-93-7
218 suppliers
$45.10/5g
28049-80-1
0 suppliers
inquiry

126726-62-3
185 suppliers
$38.50/1 g
Yield:126726-62-3 66%
Reaction Conditions:
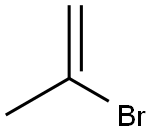
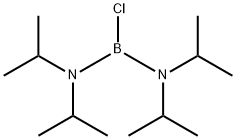
Stage #1:2-bromoprop-1-ene;bis(diisopropylamino)chloroborane with lithium in tetrahydrofuran at -10 - 25; for 6 h;
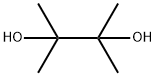
Stage #2:2,3-dimethyl-2,3-butane diol with 2,6-di-tert-butyl-4-methyl-phenol in tetrahydrofuranReflux;
Steps:
1.2 Synthesis of isopropenylboronic acid pinacol ester:
Dissolve isopropenyl bromide (89.58, 0.7411101) in 75011 ^ tetrahydrofuran, dropwise add bis (diisopropylAmine) boron chloride (183 g, 0.74 mol), lithium metal (10.4 g, 1.5 ml), and 110 mL of tetrahydrofuran were added dropwise a process controlThe reaction temperature was -10 ° C to 0 ° C. After completion of the dropwise addition, the temperature was maintained for 1 hour,The reaction was then continued for 5 hours at 25 ° C.After the completion of the reaction, pinacol (988, 0.83111 01), 2,6-di-tert-butyl-4-methylphenol (4.58) and 1201 tetrahydrofuran were added and the temperature was raised to reflux.Distillation under reduced pressure afforded the colorless transparent liquid isopropenylboronic acid pinacol ester 82.lg, yield 66%, GC: 99.7%2,6-di-tert-butyl-4-methylphenol (0.45 g) was added for long-term storage.
References:
Cangzhou Puri Oriental Technology Co., Ltd.;Leng, Yanguo;Liu, Jinzhou;Feng, Xuemin CN105503923, 2016, A Location in patent:Paragraph 0018

557-93-7
218 suppliers
$45.10/5g
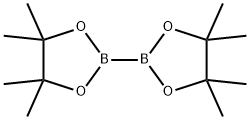
73183-34-3
581 suppliers
$6.00/5g

126726-62-3
185 suppliers
$38.50/1 g

76-09-5
385 suppliers
$8.19/250mg
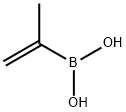
14559-87-6
62 suppliers
inquiry

126726-62-3
185 suppliers
$38.50/1 g