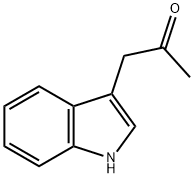
Indole-3-acetone synthesis
- Product Name:Indole-3-acetone
- CAS Number:1201-26-9
- Molecular formula:C11H11NO
- Molecular Weight:173.21
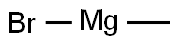
75-16-1
269 suppliers
$12.00/10ml
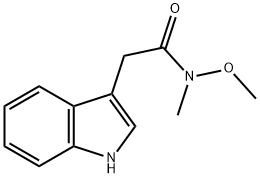
132922-37-3
28 suppliers
$45.00/10mg

1201-26-9
139 suppliers
$80.00/250mg
Yield:1201-26-9 2.35 g
Reaction Conditions:
in tetrahydrofuran at 0; for 5 h;
Steps:
a Intermediate 20. 6-Bromo-8-iodo-1,3-dimethyl-9H-pyrido[3,4-b]indole
a) 1-(1H-Indol-3-yl)propan-2-one Under argon 2-(1H-indol-3-yl)-N-methoxy-N-methylacetamide (3.00 g, 13.75mmol) was dissolved in THF (60 ml) and the solution cooled to 0 °C. A methylmagnesium bromide solution in THF(27.49 ml, 27.49 mmol) was slowly added with stirring. After 2 h a second portion of methylmagnesium bromidesolution (27.49 ml, 27.49 mmol) and after 3 h a third portion of methylmagnesium bromide solution (27.49 ml, 27.49mmol) were added. Then an aqueous ammonium chloride solution was added, followed by EA. The phases wereseparated, and the organic phase was washed with water and brine, dried, filtered and concentrated in vacuo. Theresidue was purified by chromatography over silica gel with HEP/EA (gradient) to yield 2.35 g of the title compound.LC/MS (Method LC5): RT = 1.56 min; m/z = 174.1 [M+H]+
References:
EP3318563,2018,A1 Location in patent:Paragraph 0155
4771-72-6
1 suppliers
inquiry

1201-26-9
139 suppliers
$80.00/250mg
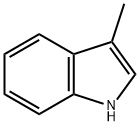
83-34-1
493 suppliers
$12.00/10g

75-36-5
580 suppliers
$17.92/100G

1201-26-9
139 suppliers
$80.00/250mg
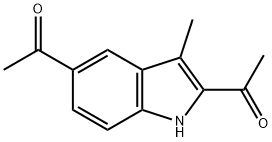
16244-29-4
0 suppliers
inquiry
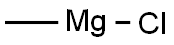
676-58-4
286 suppliers
$15.00/25ml

132922-37-3
28 suppliers
$45.00/10mg

1201-26-9
139 suppliers
$80.00/250mg
![1H-Indole, 3-[(1Z)-2-nitro-1-propen-1-yl]-](/CAS/20210305/GIF/84584-89-4.gif)
84584-89-4
1 suppliers
inquiry

1201-26-9
139 suppliers
$80.00/250mg