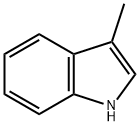
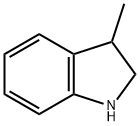
3-Methylindole synthesis
- Product Name:3-Methylindole
- CAS Number:83-34-1
- Molecular formula:C9H9N
- Molecular Weight:131.17
Industrial synthesis of 3-Methylindole: Propionaldehyde phenylhydrazone is obtained by heating propionaldehyde and phenylhydrazine to remove water molecules, and then heating with zinc chloride or sulfuric acid to remove ammonia molecules to obtain 3-Methylindole.
3 Step Synthesis of 3-Methylindole (Skatole) via Hydroboration and Cyclization

487-89-8
599 suppliers
$6.00/10g

83-34-1
493 suppliers
$12.00/10g
Yield:83-34-1 99%
Reaction Conditions:
with palladium 10% on activated carbon;water;hydrazine hydrate in ethanol at 100; for 24 h;Reagent/catalyst;
Steps:
The Typical Procedure for Pd/C Catalyzed Reduction of Carbonyls
General procedure: A: A mixture of ketone or aldehyde 1 (0.50 mmol), Pd/C (10.0 mg, 10 wt% palladium on activated carbon paste and 50% moisture, 0.9 mol% [Pd] based on starting material 1) in ethanol/water (30/1, 3 mL) was added into a Schlenk flask (25 mL) and stirred at room temperature. Then N2H4·H2O (100 mg, 2.0 mmol, 4.0 equiv) was added and the mixture was stirred at 100 °C. After the reaction was finished, the solvent was evaporated under reduced pressure and the residue was purified by column chromatography (petroleum ether/ethyl acetate 50:1 to 20:1) to provide hydrocarbons 2. B: A mixture of ketone or aldehyde 1 (0.50 mmol), Pd/C (10.0 mg, 10 wt% palladium on activated carbon paste and 50% moisture, 0.9 mol% [Pd] based on starting material 1) in ethanol (3 mL) was added into a Schlenk flask (25 mL) and stirred at room temperature. The reaction system was replaced 3 times with nitrogen and filled with hydrogen at 1 atmospheric pressure. Then the mixture was stirred at 100 °C. After the reaction was finished and released of the hydrogen, the solvent was evaporated under reduced pressure and the residue was purified by column chromatography (petroleum ether/ethyl acetate 50:1 to 20:1) to provide hydrocarbons 2. C: A mixture of ketone or aldehyde 1 (0.50 mmol), Pd/C (10.0 mg, 10 wt% palladium on activated carbon paste and 50% moisture, 0.9 mol% [Pd] based on starting material 1) in ethanol (3 mL) was added into a Schlenk flask (25 mL) and stirred at room temperature. Then ammonium formate (126 mg, 2.0 mmol, 4.0 equiv) was added and the mixture was stirred at 100 °C. After the reaction was finished, the solvent was evaporated under reduced pressure and the residue was purified by column chromatography (petroleum ether/ethyl acetate 50:1 to 20:1) to provide hydrocarbons 2.
References:
Zhou, Xiao-Yu;Chen, Xia [Tetrahedron Letters,2020,vol. 61,# 5,art. no. 151447] Location in patent:supporting information
36797-43-0
1 suppliers
inquiry

83-34-1
493 suppliers
$12.00/10g
52562-19-3
70 suppliers
$112.00/5g

83-34-1
493 suppliers
$12.00/10g
5311-88-6
1 suppliers
inquiry

83-34-1
493 suppliers
$12.00/10g
4375-15-9
45 suppliers
$53.00/100mg

83-34-1
493 suppliers
$12.00/10g