Ifosfamide synthesis
- Product Name:Ifosfamide
- CAS Number:3778-73-2
- Molecular formula:C7H15Cl2N2O2P
- Molecular Weight:261.09

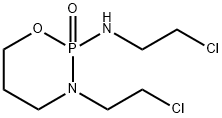
29102-47-4
5 suppliers
inquiry

3778-73-2
421 suppliers
$5.00/50mg
Yield:3778-73-2 78%
Reaction Conditions:
with hydrogenchloride in chloroform
Steps:
V 2-(2-chloroethylamino)-2-oxo-3-(2-chloroethyl)-1.3.2-oxazaphosphorinane
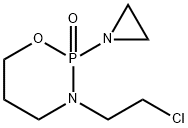
EXAMPLE V 2-(2-chloroethylamino)-2-oxo-3-(2-chloroethyl)-1.3.2-oxazaphosphorinane Into the stirred solution of 2-ethyleneimino-2-oxo-3-(2-chloroethyl)-1.3.2-oxazaphosphorinane (1.12 g, 5.0 mM) in chloroform (12 ml) was added at room temperature dropwise 3% aq. solution of hydrogen chloride (6 mL, 5.0 mM). Stirring was continued for 15 min, organic layer separated and aquous layer extracted with chloroform (2*6 mL). Organic layers were combined together, dried over anhydrous MgSO4. Removal of solvent under reduced pressure left a residue which upon crystallization from ether-n-pentane gave 1.02 g of crystalline product, mp. 45°-46° C. in 78% yield. δ31P =11.3 ppm (CHCl3). Mass spectrum m/e: 262 (0.3%), 260 (0.3%), 260 (0.8%), 213 (37%), 211 (100%).
References:
Polska Akademia Nauk-Centrum Baden Molekularnych i Makromolekularnych US4684742, 1987, A
81485-04-3
9 suppliers
inquiry

3778-73-2
421 suppliers
$5.00/50mg
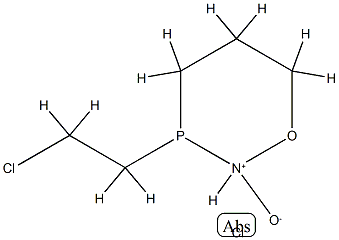
31190-87-1
0 suppliers
inquiry

3778-73-2
421 suppliers
$5.00/50mg
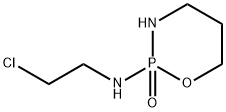
36761-83-8
24 suppliers
$65.00/1 mg

3778-73-2
421 suppliers
$5.00/50mg
![3-(2-Chloroactyl)-2-[(2-chloroethyl)amino]tetrahydro-2H-1,3,2-oxazaphosphorine-2-oxide](/CAS/GIF/72578-71-3.gif)
72578-71-3
80 suppliers
$150.00/250mg

3778-73-2
421 suppliers
$5.00/50mg