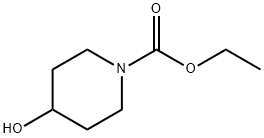
Ethyl 4-hydroxypiperidine-1-carboxylate synthesis
- Product Name:Ethyl 4-hydroxypiperidine-1-carboxylate
- CAS Number:65214-82-6
- Molecular formula:C8H15NO3
- Molecular Weight:173.21
Yield:65214-82-6 84%
Reaction Conditions:
with triethylamine in dichloromethane at 0; for 0.5 h;
Steps:
6.4.2. General procedure for the preparation of carbamates 2a-e
General procedure: To a solution of 4-hydroxy piperidine (1.0 equivalent) in dichloromethane,triethylamine (1.5 equivalent) was added at 0 C followed bydropwise addition of chloroformates (methyl, ethyl, isobutyl andbenzyl) (1.2 equivalent). After stirring for 30 min at 0 C, the reactionmixture was poured into water and extracted with dichloromethane.The organic extracts were washed with water, brine and dried over sodiumsulfate. Then organic extract was concentrated under reducedpressure to yield product with quantitative yield.
References:
Pola, Suresh;Shah, Shailesh R.;Pingali, Harikishore;Zaware, Pandurang;Thube, Baban;Makadia, Pankaj;Patel, Hoshang;Bandyopadhyay, Debdutta;Rath, Akshyaya;Giri, Suresh;Patel, Jitendra H.;Ranvir;Sundar;Patel, Harilal;Kumar, Jeevan;Jain, Mukul R. [Bioorganic and Medicinal Chemistry,2021,vol. 35,art. no. 116071]
29976-53-2
382 suppliers
$5.00/5g

65214-82-6
280 suppliers
$10.00/5g

